Abstract
Objective
To study the clinical significance and relevant factors of radiation-induced intratumoral necrosis (RIN) and peritumoral edema (PTE) after Gamma knife radiosurgery (GKRS) for intracranial meningiomas.
Methods
We retrospectively analyzed the data of 64 patients who underwent GKRS for intracranial meningioma. The mean lesion volume was 4.9 cc (range, 0.3-20), and the mean prescription dose of 13.4 Gy (range, 11-18) was delivered to the mean 49.9% (range, 45-50) isodose line. RIN was defined as newly developed or enlarged intratumoral necrosis after GKRS.
Results
RIN and new development or aggravation of PTE were observed in 21 (32.8%) and 18 (28.1%) cases of meningioma, respectively during the median follow-up duration of 19.9±1.0 months. Among various factors, maximum dose (>25 Gy) and target volume (>4.5 cc) were significantly related to RIN, and RIN and maximum dose (>24 Gy) were significantly related to the development or aggravation of PTE. In 21 meningiomas with development of RIN after GKRS, there was no significant change of the tumor volume itself between the times of GKRS and RIN. However, the PTE volume increased significantly compared to that at the time of GKRS (p=0.013). The median interval to RIN after GKRS was 6.5±0.4 months and the median interval to new or aggravated PTE was 7.0±0.7 months.
Surgical resection has been the treatment of choice for symptomatic meningiomas1,20,24). Complete resection is often curative for benign meningiomas and the progression-free survival of approximately 95% at 5 years and 90% at 10 years has been reported22,23). However, meningiomas that envelop critical neural or vascular structures cannot be completely resected due to post-resective neurological deterioration. Over the past two decades, radiosurgery has been conducted as an adjuvant or alternative procedure to resective surgery1). Gamma knife radiosurgery (GKRS) can be considered for a tumor that is not suitable for resection because GKRS has shown effective local tumor control rates and acceptable complication rates comparable to those achieved with surgical resections13,14,21). However, radiation-induced complications, such as aggravation of pre-existing peritumoral edema (PTE) have also been reported to cause neurological deterioration2). Radiation-induced intratumoral necrosis (RIN) is one of the signs of treatment response after radiosurgery, however, it can exacerbate the mass effect especially when the tumor is located adjacent to critical structures or the cerebrospinal fluid pathway until the tumor starts to shrink by RIN. Until now, relatively little has been published regarding RIN or PTE after GKRS in patients with intracranial meningiomas. Therefore, we aimed to study the incidence, clinical significance, and relevant factors of RIN and PTE during the early period after GKRS for intracranial meningiomas.
Sixty-four patients with a single intracranial meningioma underwent GKRS in our hospital during the period between August 2008 and January 2011. We retrospectively reviewed the clinical records, radiological and dosimetric data of those patients. The patients were comprised of 18 men and 46 women. The mean age at the time of GKRS was 59.7 years (range, 29-90). Among the 64 patients, GKRS was performed as a primary treatment modality in 50 patients, and as an adjunctive therapy after resective surgery for WHO grade I meningioma in 14 patients (7 for the residual, 7 for the recurrent tumor). Among 64 meningiomas, 34 lesions were located in the non-skull base and 30 in the skull base. The locations were the convexity (n=22), petrous apex (n=12), falx (n=7), olfactory groove (n=6), sphenoid wing (n=6), parasagittal (n=5), cavernous sinus (n=5), and tentorium (n=1).
GKRS was performed using a model C Leksell Gamma Knife (Elekta Instruments AB, Stockholm, Sweden). The planning system was a Leksell Gamma Plan version 8.3.1 (Elekta Instruments AB). For magnetic resonance (MR) imaging of radiosurgery planning, T1-weighted axial images with contrast and T2-weighted axial images were obtained with 2 mm slice thickness without gaps. The mean volume of 64 meningiomas was 4.9 cc (range, 0.3-20). The mean prescription dose of 13.4 Gy (range, 11-18) was delivered to the mean 49.9% (range, 45-50) isodose line. The prescribed dose was determined according to the tumor volume. Tumors with a volume of <1 cc, 1-5 cc, 5-10 cc and >10 cc were treated with 18-16 Gy, 16-14 Gy, 14-13 Gy and 13-11 Gy, respectively, until September 2009. However, the prescription dose was lowered starting October 2009 because of severe PTE in our cases. Tumors with a volume of <1 cc, 1-5 cc, 5-10 cc and >10 cc were treated with 14 Gy, 13 Gy, 12 Gy and 11 Gy, respectively, according to the literature6). The mean volume coverage was 96.4% (range, 84-99). The radiosurgical prescription parameters (RPP) evaluated were Paddick's conformity index (CI), Shaw's CI, and gradient index (GI)18,25).
MR imaging was performed every 6 months, including continuous thin cut T1 enhanced images, which was the same technique as MR imaging for GKRS. Tumor volume was calculated from enhancing lesions in T1 enhanced images, and PTE volume was calculated from T2 abnormal signal volume minus the tumor volume. Volume measurements of tumors and PTE were performed using the co-registration program (Leksell Gamma Plan®, version 8.3.1). Local tumor control was assessed according to the Macdonald's criteria9,17). Complete response (CR) was defined as a complete disappearance of all enhancing tumors, partial response (PR) as ≥50% decrease in enhancing tumor volume, progressive disease (PD) as ≥25% increase in enhancing tumor volume, and stable disease (SD) as <50% decrease or <25% increase in enhancing tumor volume. We defined local tumor control as CR, PR, and SD. Intratumoral necrosis was defined as low signal intensity area on contrast-enhanced T1-weighted images7,8). Radiation-induced intratumoral necrosis (RIN) was defined as newly developed intratumoral necrosis or aggravation of pre-existing necrosis of more than 25% on the follow-up imaging after GKRS. Aggravation of pre-existing PTE was defined as at least 25% increase of the volume, compared to the baseline data measured at the time of GKRS.
Statistical analysis was performed using SPSS version 12.0 (SPSS, Chicago, IL, USA). Local control rate was calculated from the time of GKRS. To investigate relevant factors, Kaplan-Meier analysis was used for categorical variables, and Cox regression model was used for continuous variables and multivariate analysis. Results were regarded as significant for p<0.05.
The median clinical follow-up duration was 19.9 months (range, 3.7-35.1). Sixty-four meningiomas were assessed by at least one follow-up imaging with a mean imaging follow-up duration of 12.9 months (range, 5.4-33.4).
Results of local tumor control at the time of the last follow-up were CR in 0 (0%), PR in 3 (4.7%), SD in 60 (93.7%), and PD in 1 (1.6%). The one progressed case was not a true failure because RIN was observed at the last follow-up (17.8 months after GKRS) and tumor shrinkage is expected with further follow-up. The actuarial local tumor control rate at 5 years was 97.9%13).
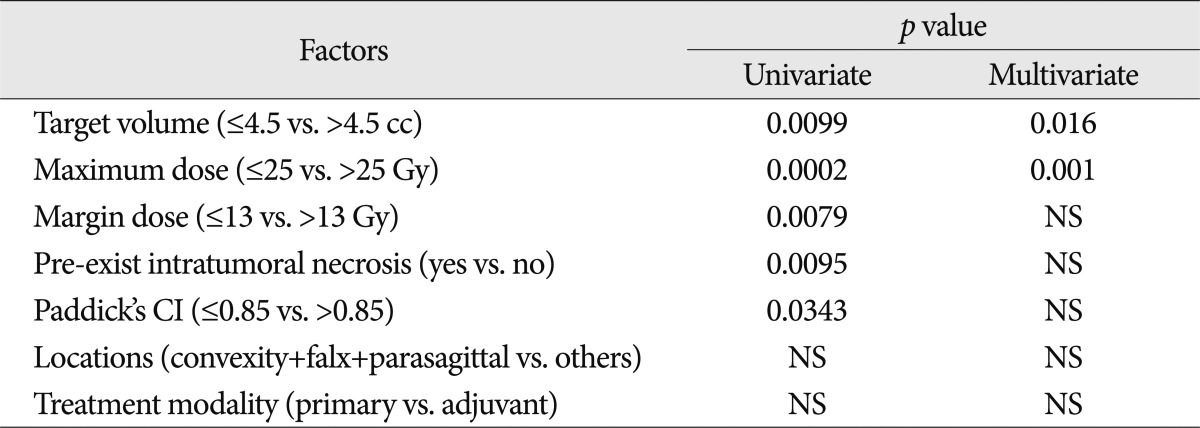
At the time of GKRS, intratumoral necrosis was observed in 5 (7.8%) out of 64 meningiomas but RIN after GKRS was observed in 21 (32.8%) lesions. Among 21 lesions, new radiation-induced intratumoral necrosis developed in 16 (76.2%), and 5 (23.8%) showed aggravation of pre-existing intratumoral necrosis of more than 25%. However, there was no significant change of tumor volume itself between the time of GKRS and RIN (mean, 7.0 vs. 7.8 cc). The median interval to the development of RIN was 6.5±0.4 months (range, 3.7-17.0). Age, Paddick's CI, marginal dose, maximum dose, target volume, locations, pre-existing intratumoral necrosis and treatment modality were assessed for factors related to RIN. Target volume >4.5 cc (p=0.0099), margin dose >13Gy (p=0.0079), maximum dose >25 Gy (p=0.0002), Paddick's CI ≤0.85 (p=0.0343) and pre-existing intratumoral necrosis (yes) (p=0.0095) were significant factors related to RIN in univariate analysis. Both the maximum dose >25 Gy (p=0.001, odds ratio=6.313, 95% confidence interval : 2.089-19.080 using the forward stepwise method) and target volume >4.5 cc remained significant in multivariate analysis (p=0.016, odds ratio=0.287, 95% confidence interval : 0.104-0.794 using the forward stepwise method) (Table 1). RIN rates of target volume >4.5 cc were 3.3%, 10.0% and 46.7% at 6, 12 and 24 months, respectively. However, RIN rates of target volume ≤4.5 cc were 2.3%, 2.3% and 13.6% at 6, 12 and 24 months, respectively.
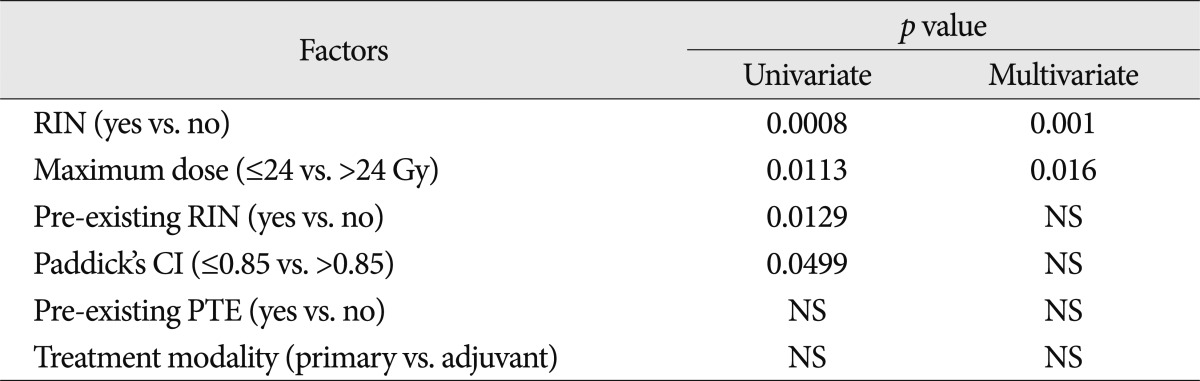
PTE was observed in 11 (17.2%) out of 64 meningiomas at the time of GKRS, but in 18 lesions (28.1%) after GKRS during the follow-up period. Among 18 lesions, PTE was newly developed in seven lesions and six showed aggravation of pre-existing PTE, meaning that new or aggravated PTE was observed in 20.3% (13/64) of meningiomas. The median interval to new development or aggravation of PTE was 7.0±0.7 months (range, 1.1-13.1), which was a little later than the median interval to RIN. Among the factors, RIN (yes) (p=0.0008), Paddick's CI ≤0.85 (p=0.0449), pre-existing RIN (yes) (p=0.0129) and maximum dose >24 Gy (p=0.0113) were the significant factors related to new or aggravated PTE in univariate analysis. Both RIN (yes) (p=0.001, odds ratio=0.086, 95% confidence interval : 0.019-0.387 using the forward stepwise method) and maximum dose >24 Gy (p=0.016, odds ratio=10.000, 95% confidence interval : 1.535-65.137 using the forward stepwise method) also remained significant in multivariate analysis. However, pre-existing PTE, target volume and marginal dose were not significantly related to new or aggravated PTE in univariate and multivariate analyses (Table 2).
Among 21 lesions with developed RIN after GKRS, PTE was observed in 7 lesions at the time of GKRS and in 14 after GKRS. The mean volume of 7 pre-existing PTE was 8.7 cc (range, 0.1-17.5), however, the mean volume of 14 PTE after GKRS was significantly increased to 15.5 cc (range, 0.8-38.6) (p=0.013, Wilcoxon signed ranks test).
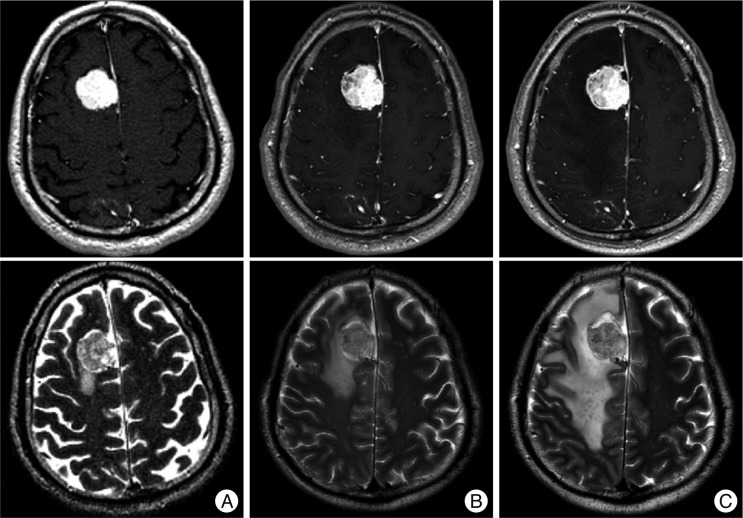
Among 21 lesions that showed RIN with or without PTE and seven lesions that showed PTE without RIN, 9 lesions were symptomatic during the median follow-up period of 18.2±2.4 months (range, 6.8-29.4) after GKRS. Therefore, symptomatic complications developed during the early period after GKRS in 14.1% (9/64) of the patients in our series. RIN or PTE-related symptoms were headache in 4, dizziness in 3 and seizure in 2. The median onset time was 6.0±0.3 months (range, 3.7-13.5) after GKRS. Among 9 patients, symptoms resolved in six patients after the median time of 5.4 months (range, 0.6-12.2) but the symptoms continued in two patients despite steroid administration. Decompressive surgery was required in one patient because of severe PTE. A 51-year old male patient underwent GKRS for a meningioma of 15.4 cc in volume with a prescription dose of 13 Gy at 50% isodose line. The PTE volume at the time of GKRS was 6.3 cc. The patient complained of dizziness and the follow up MR imaging taken at 5.6 months after GKRS showed RIN and aggravation of PTE. Even after administration of oral steroid, a follow-up MR taken at 11.8 months after GKRS showed a further increase of tumor volume and PTE to 18.7 cc and 38.6 cc, respectively (Fig. 1). His neurological condition deteriorated rapidly, and decompressive surgery was performed eventually. Pathologic examination showed the characteristics of multifocal necrosis.
Stereotactic radiosurgery offers a relatively non-invasive and effective method in the management for intracranial tumors, including meningiomas14). Metellus et al.15) compared GKRS and conventional radiotherapy for primary and residual meningiomas. They concluded that both conventional radiotherapy and GKRS were safe and efficient, but GKRS provided a better radiological response, and was more compatible with most patients than conventional radiotherapy. Hsieh et al.10) compared the treatment results of meningioma between GKRS and linear accelerator-based radiosurgery. They reported that the mean complication rate was 15.2% (range, 8.3-23.6) in the GKRS treated group treated, and 25.9% (range, 17.0-34.9) in those treated using a linear accelerator. Therefore, GKRS can be said to provide more beneficial effects in terms of radiation-induced complications. Radiation-induced imaging changes can be the signs showing treatment response after GKRS, but these changes can also exacerbate patients' symptoms or neurological signs by increasing the mass effect. These unwanted effects can prompt additional treatments, such as steroid administration or decompressive surgery, especially in benign tumors. Therefore, some researchers have studied radiation-induced imaging changes after stereotactic radiosurgery for benign intracranial tumors and relevant factors. Chang et al.3) reported that patients treated with GKRS for meningiomas experienced peritumorous imaging changes at a rate of 23.6%. In their series, tumor location, maximal dose, and margin dose were related to the occurrence of imaging changes after GKRS in univariate analysis. However, only tumor location (convexity, parasagittal and falx cerebri) remained significant in multivariate analysis. Other studies have reported regarding the significance of tumor location and the location of the non-skull base meningiomas seemed to encounter more adverse radiation effects compared to the skull base lesions4,5,12,16,19). However, there was no correlation between tumor location and adverse radiation effects in our study.
Among the imaging changes that can lead to clinical deterioration after GKRS, PTE has been the most frequently studied. Cai et al.2) reported that worsened pre-existing PTE, or new PTE occurred in about 25% of patients who underwent GKRS for intracranial meningiomas. Tumor-brain contact interface area was a strong predictor for the occurrence of new PTE in their series. Kollová et al.13) analyzed 400 meningiomas and found that tumors with edema before GKRS, tumor volume >10.0 cc, tumors treated with a maximum dose >30 Gy and margin dose >16 Gy were significantly related to post-GKRS edema (aggravation or new development). We thought that RIN may lead to neurological deterioration because RIN can result in transient tumor volume increase, especially when meningiomas are located adjacent to the critical structures, such as the cerebrospinal fluid pathway. In our series, a target volume >4.5 cc and maximum dose >25 Gy were significantly related to the development of RIN, but there was no significant increase of tumor volume itself in the 21 meningiomas that developed RIN after GKRS. However, RIN was significantly related to new development or aggravation of PTE in both univariate and multivariate analyses. The median interval to the development of RIN (6.5±0.4 months) was a little shorter compared to that of PTE (7.0±0.7 months). These results may suggest that RIN can be a warning sign of the development or aggravation of PTE.
Besides RIN, maximum dose >24 Gy was also a significant factor related to new development or aggravation of PTE in our study. Flickinger et al.6) reported on the possible relations between marginal dose and complications occurring after GKRS of meningiomas. They reported that the actuarial rates of any symptomatic post-radiosurgical sequelae (at 10 years) were 5.3±2.3% in the median marginal dose 14 Gy (range, 8.9-20) and 22.9±9.3% in the median marginal dose 17 Gy (range, 10-20). In addition, they insisted that complications correlated with the volume of tissue receiving ≥12 Gy. However, marginal dose was not significantly related to new development or aggravation of PTE in our study. This result may be caused by the lowered prescription dose from October 2009 after having patients experience severe PTE after GKRS. Although prescription dose is a fixed value, maximum dose can be varied. Hur et al.11) reported on the difference of the maximum dose ranges from 0.7-7% by matrix size and target volume. Their results are coherent with our results which showed that maximum dose was significantly related to new development or aggravation of PTE, instead of marginal dose. Among 64 lesions, 11 lesions were treated with marginal dose of 12 Gy showed the range of maximum dose from 23.6-24.3 Gy (mean, 24.1) in our study.
In our series, target volume >4.5 cc and maximum dose >25 Gy were significant factors for the development of RIN after GKRS for intracranial meningiomas. RIN and maximum dose >24 Gy were significantly related to new development or aggravation of PTE, and RIN preceded new development or aggravation of PTE. Therefore, we suggest that close observation is required for meningiomas treated with a maximum dose >24 Gy and showing RIN after GKRS, because accompanying PTE may deteriorate neurological conditions when they are located adjacent to critical structures or the cerebrospinal fluid pathway.
References
1. Alexiou GA, Gogou P, Markoula S, Kyritsis AP. Management of meningiomas. Clin Neurol Neurosurg. 2010; 112:177–182. PMID: 20056312.

2. Cai R, Barnett GH, Novak E, Chao ST, Suh JH. Principal risk of peritumoral edema after stereotactic radiosurgery for intracranial meningioma is tumor-brain contact interface area. Neurosurgery. 2010; 66:513–522. PMID: 20173546.

3. Chang JH, Chang JW, Choi JY, Park YG, Chung SS. Complications after gamma knife radiosurgery for benign meningiomas. J Neurol Neurosurg Psychiatry. 2003; 74:226–230. PMID: 12531956.

4. Chung HT, Kim DG, Paek SH, Jung HW. Development of dose-volume relation model for gamma knife surgery of non-skull base intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2009; 74:1027–1032. PMID: 19056186.

5. El Shehaby A, Ganz JC, Reda WA, Hafez A. Mechanisms of edema after gamma knife surgery for meningiomas. Report of two cases. J Neurosurg. 2005; 102(Suppl):1–3. PMID: 15662770.
6. Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys. 2003; 56:801–806. PMID: 12788188.

7. Goodman KA, Sneed PK, McDermott MW, Shiau CY, Lamborn KR, Chang S, et al. Relationship between pattern of enhancement and local control of brain metastases after radiosurgery. Int J Radiat Oncol Biol Phys. 2001; 50:139–146. PMID: 11316557.

8. Goyal S, Prasad D, Harrell F Jr, Matsumoto J, Rich T, Steiner L. Gamma knife surgery for the treatment of intracranial metastases from breast cancer. J Neurosurg. 2005; 103:218–223. PMID: 16175849.

9. Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol. 2008; 29:419–424. PMID: 18272557.

10. Hsieh CT, Tsai JT, Chang LP, Lin JW, Chang SD, Ju DT. Peritumoral edema after stereotactic radiosurgery for meningioma. J Clin Neurosci. 2010; 17:529–531. PMID: 20116255.

11. Hur BI, Choi BK, Sung SK, Cho WH, Cha SH, Choi CH. The variable ellipsoid modeling technique as a verification method for the treatment planning system of gamma knife radiosurgery. J Korean Neurosurg Soc. 2010; 47:128–133. PMID: 20224712.

12. Kim DG, Kim ChH, Chung HT, Paek SH, Jeong SS, Han DH, et al. Gamma knife surgery of superficially located meningioma. J Neurosurg. 2005; 102(Suppl):255–258. PMID: 15662820.

13. Kollová A, Liscák R, Novotný J Jr, Vladyka V, Simonová G, Janousková L. Gamma Knife surgery for benign meningioma. J Neurosurg. 2007; 107:325–336. PMID: 17695387.

14. Kondziolka D, Mathieu D, Lunsford LD, Martin JJ, Madhok R, Niranjan A, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008; 62:53–58. discussion 58-60. PMID: 18300891.

15. Metellus P, Regis J, Muracciole X, Fuentes S, Dufour H, Nanni I, et al. Evaluation of fractionated radiotherapy and gamma knife radiosurgery in cavernous sinus meningiomas : treatment strategy. Neurosurgery. 2005; 57:873–886. discussion 873-886. PMID: 16284558.

16. Mindermann T, de Rougemont O. The significance of tumor location for Gamma Knife treatment of meningiomas. Stereotact Funct Neurosurg. 2004; 82:194–195. PMID: 15557769.

17. Molenaar R, Wiggenraad R, Verbeek-de Kanter A, Walchenbach R, Vecht C. Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br J Neurosurg. 2009; 23:170–178. PMID: 19306173.

18. Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000; 93(Suppl 3):219–222. PMID: 11143252.

19. Pamir MN, Peker S, Kilic T, Sengoz M. Efficacy of gamma-knife surgery for treating meningiomas that involve the superior sagittal sinus. Zentralbl Neurochir. 2007; 68:73–78. PMID: 17614087.

20. Sanna M, Bacciu A, Falcioni M, Taibah A, Piazza P. Surgical management of jugular foramen meningiomas : a series of 13 cases and review of the literature. Laryngoscope. 2007; 117:1710–1719. PMID: 17690614.

21. Sheehan JP, Williams BJ, Yen CP. Stereotactic radiosurgery for WHO grade I meningiomas. J Neurooncol. 2010; 99:407–416. PMID: 20734218.

22. Shrivastava RK, Sen C, Costantino PD, Della Rocca R. Sphenoorbital meningiomas : surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005; 103:491–497. PMID: 16235682.

23. Sindou M, Wydh E, Jouanneau E, Nebbal M, Lieutaud T. Long-term follow-up of meningiomas of the cavernous sinus after surgical treatment alone. J Neurosurg. 2007; 107:937–944. PMID: 17977264.

24. Voss NF, Vrionis FD, Heilman CB, Robertson JH. Meningiomas of the cerebellopontine angle. Surg Neurol. 2000; 53:439–446. discussion 446-447. PMID: 10874142.

25. Woo HJ, Hwang SK, Park SH, Hwang JH, Hamm IS. Factors related to the local treatment failure of γ knife surgery for metastatic brain tumors. Acta Neurochir (Wien). 2010; 152:1909–1914. PMID: 20890616.

Fig. 1
MR images of a 51-year-old male patient treated with a prescription dose of 13 Gy at 50% isodose (target volume 15.4 cc, maximum dose 26.4 Gy) : T1-weighted images with double dose contrast (upper row) and T2-weighted images (lower row). A : Before Gamma knife radiosurgery (GKRS). B : 5.6 months after GKRS. C : 11.8 months after GKRS.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download