Abstract
Objective
The study examined the difference in the incidence of symptomatic cerebral vasospasm with magnesium supplementation in aneurysmal subarachnoid hemorrhage (SAH) in a Korean population.
Methods
This retrospective analysis was performed in 157 patients diagnosed with aneurysmal SAH from January 2007 to December 2011 at a single center. Seventy patients (44.6%) received a combination treatment of nimodipine with magnesium and 87 patients (55.4%) received only nimodipine. A matched case-control study using propensity scores was conducted and 41 subjects were selected from each group. A dosage of 64 mmol/day of magnesium was administrated.
Results
The infusion of magnesium did not reduce the incidence of symptomatic cerebral vasospasm (n=7, 17.1%, p=0.29) compared with simple nimodipine injection (n=11, 26.8%). The ratios of good clinical outcome (modified Rankin scale 0-2) at 6 months were similar, being 78% in the combination treatment group and 80.5% in the nimodipine only group (p=0.79). The proportions of delayed cerebral infarction was not significantly lower in patients with combination treatment (n=2, 4.9% vs. n=3, 7.3%; p=0.64). There was no difference in the serum magnesium concentrations between the patients with symptomatic vasospasm and without vasospasm who had magnesium supplementation. No major complications associated with intravenous magnesium infusion were observed.
Symptomatic cerebral vasospasm is still a leading risk factor for poor outcome in aneurysmal subarachnoid hemorrhage (SAH)7). Magnesium has been used as a neuroprotective agent for preventing vasospasm with the rationale that its vasodilatory action on vasospastic artery and improvement of cerebral blood flow result from the blockage of calcium channels and inhibition of myosin light chain kinase1,17). Glutamate interaction with N-methyl-D-asparate (NMDA) can also be prohibited, consequently leading to reduced calcium release from the intracellular space including endoplasmic reticulum, mitochondria and calciosomes, as well as inhibiting calcium influx from extracellular space19). In addition, membrane depolarization and subsequent cell swelling may be prevented by blocking sodium influx14).
Many studies have been conducted to confirm the clinical validity of magnesium treatment, but its benefits to the decrease in symptomatic vasospasm and association with good clinical outcome are controversial2,4-6,8,18,20-22,24).
Although the benefit of magnesium sulfate for cerebral vasospasm in SAH remains debatable, clinical research on the effectiveness of magnesium has not yet fully conducted in Korea. To address this shortcoming, we performed a case-control study with the use of propensity scores to compare the differences in the incidence of symptomatic vasospasm between a group treated with a combination of nimodipine and magnesium and a group treated solely with nimodipine.
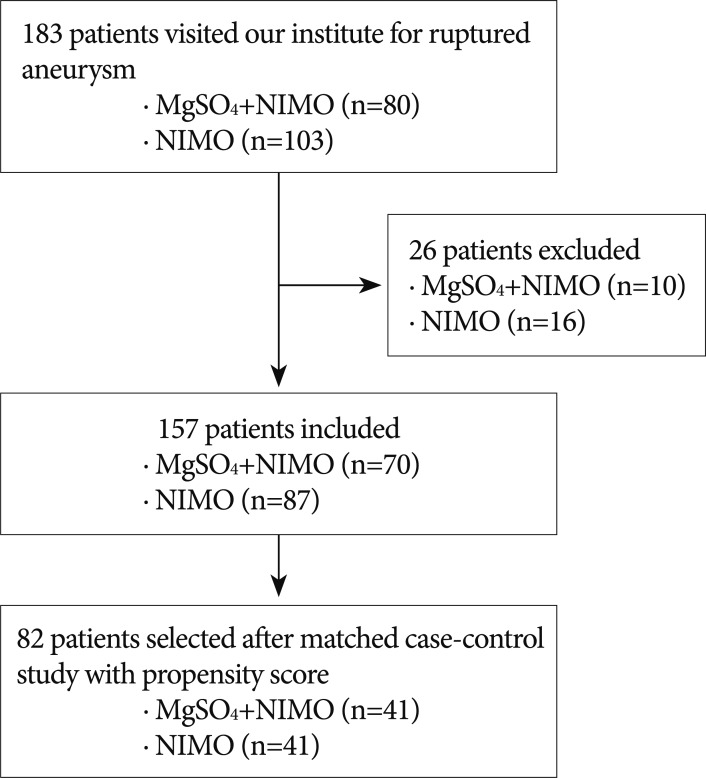
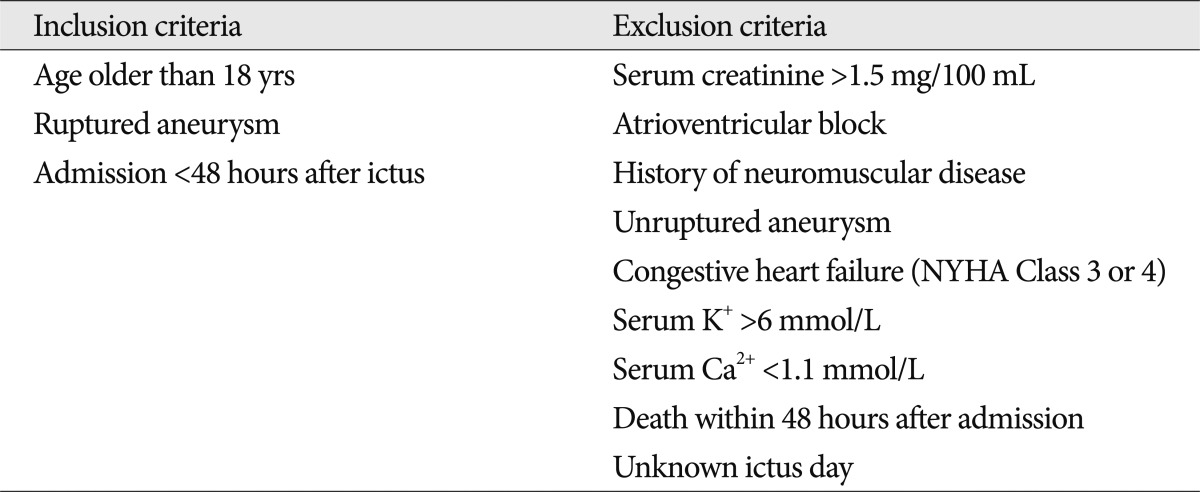
The study was approved by Institutional Review Board (IRB) of the Ethical Committee for Human Research. This retrospective analysis was performed in 183 patients who presented with ruptured aneurysms from January 2007 to December 2011 at a single center. Nimodipine was infused in 103 (56.3%) patients until October 2009 and the combination of magnesium with nimodipine was infused in 80 (43.7%) patients after November 2009. A flow diagram of the study is shown in Fig. 1. In cases of surgical complications, infection and rebleeding of an aneurysm were excluded in this study due to possibility of bias. Detailed information of inclusion and exclusion criteria is presented in Table 1. Their medical information was recorded with variables including sex, age, hypertension (HTN), diabetes mellitus (DM), Hunt and Hess grade (H-H grade), Fisher grade, method of treatment, occurrence of symptomatic cerebral vasospasm, and clinical outcome. Postoperative radiologic examinations were conducted with brain computed tomography (CT), 2012magnetic resonance imaging (MRI), or transcranial Doppler (TCD) sonography. Symptomatic cerebral vasospasm was proven through two processes. When a newly developed neurological deficit, such as deterioration in the level of consciousness, dysphasia, motor weakness, or sensory changes in patients who were capable of communication was found, it had to be confirmed by cerebral angiography. Vasospasms were not considered if they were caused by rebleeding of an aneurysm, intracerebral hemorrhage (ICH), hydrocephalus, electrolyte abnormality, ischemic events related to surgery, seizure, infection or medical causes. In cases involving patients who were not accurately evaluated by a neurologic examination, a symptomatic vasospasm was suspected when an increase of the TCD velocity (mean velocity of arterial flow over 140 cm/sec in the anterior circulation or 90 cm/sec in the basilar circulation) was seen or if a cerebral infarction was shown by CT or diffusion MRI. Next, the suspected vasospasm had to be confirmed by subsequent angiography. After symptomatic vasospasm was confirmed, a selective intra-arterial injection of nimodipine3) or a transluminal balloon angioplasty12) was performed according to the neurosurgeon's discretion and repeated several times, if necessary, in order to restore blood flow.
Clinical outcome was measured by the modified Rankin scale (mRS) 6 months after ictus. A good outcome was defined as a mRS score of 0-2 and a poor outcome was a mRS score of 3-6. Except for magnesium supplementation, the same protocol of treatment was applied to both groups including nimodipine (Samjin Pharm., Seoul, Korea). All patients received intravenous nimodipine (20 µg/kg/h) for two weeks and the treatment was changed to oral nimodipine (60 mg/4 h) until discharge.
Patients received magnesium (Daewon Pharm., Seoul, Korea) through a continuous infusion of 64 mmol/day18) for 14 days post-operatively without loading or additional supplemental magnesium. The purpose of this magnesium dosage was to maintain a serum concentration of 1.0-2.0 mmol/L with no magnesium intoxication16). Serum magnesium concentrations were measured every 2 days. The occurrence of complications such as bradycardia, hypotension, and bradypnea with depressed oxygen saturations induced by magnesium toxicity19) were closely monitored.
Continuous data are presented as the mean and standard deviation (SD). A chi-square or Student's t-test was used. A propensity score-matching analysis was conducted with the probability of magnesium injection and multivariate logistic regression analysis including relevant variables (sex, age, H-H grade, Fisher grade, and HTN) was performed to calculate a propensity score for each subject. A greedy-matching technique was applied for one-to-one match. Repeated measures analysis of variance (ANOVA), Mauchly's sphericity test, and a Lower-bound correction were used to analyze the differences in serum concentration of magnesium between patients with symptomatic vasospasm and without vasospasm who had combination treatment. p-values <0.05 were regarded as statistically significant. Matching analysis was performed with SAS (Release 9.1.3; SAS Institute, Cary, NC, USA) and other statistics were done with SPSS (version 18; SPSS, Chicago, IL, USA)
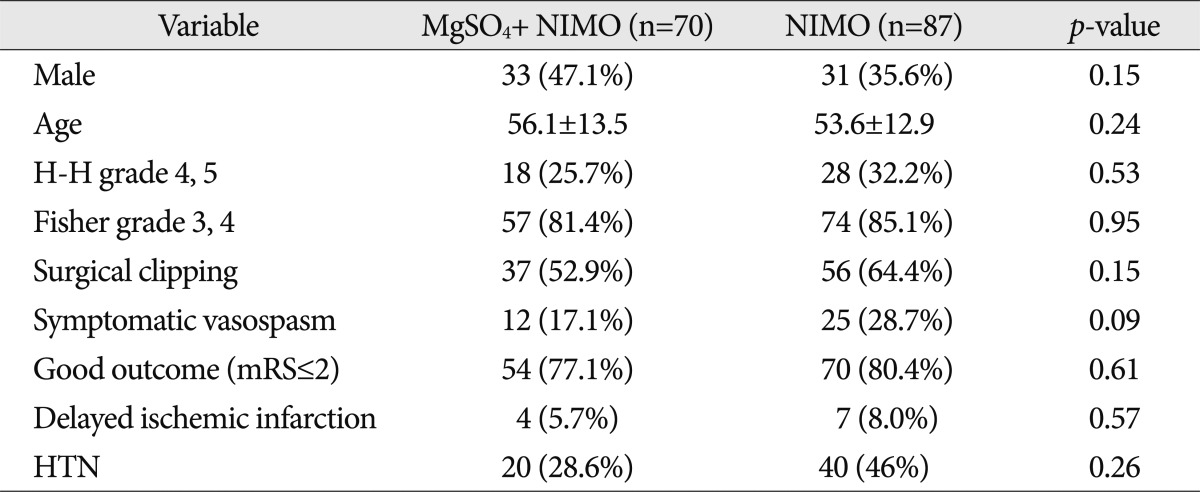
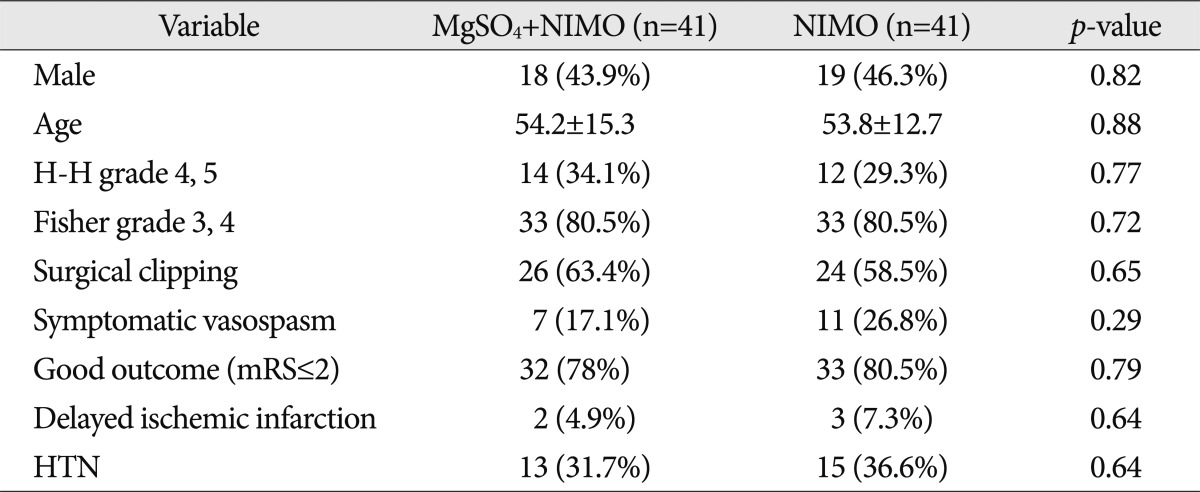
The characteristics of two groups in 157 unmatched cases are shown in Table 2. Although significant differences of the variables were not detected in both groups, results such as symptomatic vasospasm and good clinical outcome may be affected by the different proportion of the variables.
Data from a propensity score matching analysis in 82 patients are presented in Table 3. Supplementation with magnesium did not contribute to a decrease in symptomatic vasospasm. In particular, the chi-square result of the occurrence of cerebral vasospasm was higher than that of 157 unmatched cases (0.29 and 0.09, respectively).
The ratios of good clinical outcome (mRS of 0-2) at 6 months were 78% in the combination treatment group and 80.5% in nimodipine only group (p=0.79). The proportions of delayed cerebral infarction were not significantly lower in patients with combination treatment (n=2, 4.9%) compared with the nimodipine only group (n=3, 7.3%, p=0.64).
The serial magnesium concentrations between the patients with symptomatic vasospasm and without vasospasm who had magnesium supplementation are shown in Fig. 2. The normal range of magnesium was 0.7-0.95 mmol/L. There was no difference in the serum concentrations of magnesium between the two groups (p=0.50).
This study indicates that the combination treatment of 64 mmol/day of magnesium with intravenous nimodipine does not reduce the incidence of symptomatic cerebral vasospasms in ruptured aneurysms during the initial 2 weeks. The proportions of good clinical outcome and delayed cerebral infarction were similar. There was no difference in serum magnesium concentrations between the group of symptomatic vasospasm and non-vasospasm in patients with magnesium infusion.
Supplementation with intravenous magnesium is contentious. Accurate maintenance dosage and targeted serum concentration of magnesium have remained unclear. van den Bergh et al.18) suggested that a risk reduction on delayed cerebral ischemia in SAH patients could be realized using a dosage regimen of 64 mmol/day of magnesium. Friedlich et al.6) reported that continuous low doses of parenteral MgSO4 of 0.6 g/h given within 72 hours after SAH reduced the incidence of clinical and radiological vasospasm without reaching the specific serum concentrations level. Westermaier et al.21) demonstrated the benefit of high dose magnesium on good outcome and angiographic vasospasm. The authors provided intravenous magnesium to maintain a concentration of 2.0-2.5 mmol/L with a bolus injection and adjustment of magnesium every 8 hours. On the contrary, Wong et al.22,23) insisted that there was no evidence that high plasma magnesium levels could improve clinical outcome.
The ideal administration route of magnesium for preventing vasospasm has been debatable. Wong et al.22) assumed that an intracisternal infusion of magnesium needs to be adapted for a good clinical outcome due to the toxicity of high levels of magnesium concentration (over 2.5 mmol/L). Several studies also supported the effectiveness of intracisternal magnesium infusion in preventing vasospasms and they recommend maintaining levels >3 mEq/L in cerebrospinal fluid9,10,15). However, there is no appropriate explanation for the benefit of intracisternal magnesium irrigation and this may increase the infection risk. In addition, it is not suitable for patients with coil embolization to keep drainage tubes within the cerebral cisternal space.
The ideal consensus about identifying cerebral infarction caused by vasospasm remains unclear. With the advance of endovascular therapy, there are difficulties about differentiating causes of infarction, whether cerebral vasospasm or procedure-related complication. Therefore, measuring the clinical effect of magnesium supplementation on the reduction of cerebral infarction may vary among observers and it may also lead to different clinical outcome. In this study, there were fewer cases of delayed cerebral infarction compared with another study21) that was performed with high maintenance of magnesium of 2.0-2.5 mmol/L in spite of similar rate of high grade Hunt and Hess and Fisher grade. This difference may have reflected differences in the endovascular treatment and may have caused opposite results.
The combined mechanisms of magnesium with nimodipine are still not well understood. Although nimodipine and magnesium act through calcium-dependent channels, their synergic pathway has not been clarified.
Finally, the optimal time of the start of magnesium has not been well established. In acute stroke, infusion within 12 h has not proven to be beneficial to the risk reduction of patients with poor outcomes11). However, these results may not apply to patients with ruptured aneurysms because of the differences of pathophysiology and drug regimen. An on-going study [Field Administration of Stroke Therapy-Magnesium (FAST-MAG) trial] seeks to assess the advantage of earlier magnesium infusion within the first 2 hours. The results will require confirmation.
Limitations of the study include study design and the magnesium dosage. This was a small retrospective study. However, the measured variables of patients have a lesser proportional difference in risk factors related to cerebral vasospasm between two groups with the use of the propensity score matching. Rubin and Thomas13) reported propensity score matching analysis has lesser error estimates than simple multiple regression analysis. Although a high concentration of serum magnesium level of more than 2.5 mmol/L may reduce symptomatic cerebral vasospasm22), but it may not be appropriate considering the higher chance of magnesium toxicity.
Combination treatment of 64 mmol/day of magnesium with nimodipine may not be beneficial to the reduction of the incidence of symptomatic cerebral vasospasm and the improvement of clinical outcome in patients with ruptured aneurysmal SAH.
However, the beneficial effect of magnesium in preventing delayed cerebral infarction or improving neurological recovery in SAH cannot be excluded. Therefore, a nationwide randomized controlled multicenter trial of intravenous magnesium sulfate in aneurysmal SAH including appropriate dosage, targeted serum concentration of magnesium, initiation or maintenance time period is needed in Korea.
References
1. Altura BT, Altura BM. Interactions of Mg and K on cerebral vessels--aspects in view of stroke. Review of present status and new findings. Magnesium. 1984; 3:195–211. PMID: 6399342.
2. Barbarawi M, Smith SF, Jamous MA, Haboub H, Suhair Q, Abdullah S. Therapeutic approaches to cerebral vasospasm complicating ruptured aneurysm. Neurol Int. 2009; 1:e13. PMID: 21577350.

3. Biondi A, Ricciardi GK, Puybasset L, Abdennour L, Longo M, Chiras J, et al. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage : preliminary results. AJNR Am J Neuroradiol. 2004; 25:1067–1076. PMID: 15205150.
4. Boet R, Mee E. Magnesium sulfate in the management of patients with Fisher Grade 3 subarachnoid hemorrhage : a pilot study. Neurosurgery. 2000; 47:602–606. discussion 606-607. PMID: 10981747.

5. Collignon FP, Friedman JA, Piepgras DG, Pichelmann MA, McIver JI, Toussaint LG 3rd, et al. Serum magnesium levels as related to symptomatic vasospasm and outcome following aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2004; 1:441–448. PMID: 16174947.

6. Friedlich D, Agner C, Boulos AS, Mesfin F, Feustel P, Bernardini GL, et al. Retrospective analysis of parenteral magnesium sulfate administration in decreased incidence of clinical and neuroradiological cerebral vasospasm : a single center experience. Neurol Res. 2009; 31:621–625. PMID: 19660191.

7. Keyrouz SG, Diringer MN. Clinical review : prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit Care. 2007; 11:220. PMID: 17705883.

8. Macdonald RL, Curry DJ, Aihara Y, Zhang ZD, Jahromi BS, Yassari R. Magnesium and experimental vasospasm. J Neurosurg. 2004; 100:106–110. PMID: 14743919.

9. Mori K, Yamamoto T, Miyazaki M, Hara Y, Aiko Y, Koike N, et al. Optimal cerebrospinal fluid magnesium ion concentration for vasodilatory effect and duration after intracisternal injection of magnesium sulfate solution in a canine subarachnoid hemorrhage model. J Neurosurg. 2011; 114:1168–1175. PMID: 21073257.

10. Mori K, Yamamoto T, Nakao Y, Osada H, Hara Y, Oyama K, et al. Initial clinical experience of vasodilatory effect of intra-cisternal infusion of magnesium sulfate for the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo). 2009; 49:139–144. discussion 144-145. PMID: 19398856.

11. Muir KW, Lees KR, Ford I, Davis S. Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial : randomised controlled trial). Lancet. 2004; 363:439–445. PMID: 14962524.
12. Polin RS, Coenen VA, Hansen CA, Shin P, Baskaya MK, Nanda A, et al. Efficacy of transluminal angioplasty for the management of symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2000; 92:284–290. PMID: 10659016.

13. Rubin DB, Thomas N. Combining propensity score matching with additional adjustments for prognostic covariates. J Am Stat Assoc. 2000; 95:573–585.

14. Sang N, Meng Z. Blockade by magnesium of sodium currents in acutely isolated hippocampal CA1 neurons of rat. Brain Res. 2002; 952:218–221. PMID: 12376182.

15. Satoh A, Sugiyama T, Ooigawa H, Nakajima H, Ogura T, Neki H, et al. Prevention of symptomatic vasospasm by continuous cisternal irrigation with mock-CSF containing ascorbic acid and Mg 2. Acta Neurochir Suppl. 2010; 107:115–118. PMID: 19953382.
16. van den Bergh WM, Albrecht KW, Berkelbach van der Sprenkel JW, Rinkel GJ. Magnesium therapy after aneurysmal subarachnoid haemorrhage a dose-finding study for long term treatment. Acta Neurochir (Wien). 2003; 145:195–199. discussion 199. PMID: 12632115.

17. van den Bergh WM, Algra A, van der Sprenkel JW, Tulleken CA, Rinkel GJ. Hypomagnesemia after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003; 52:276–281. discussion 281-282. PMID: 12535355.

18. van den Bergh WM, Algra A, van Kooten F, Dirven CM, van Gijn J, Vermeulen M, et al. Magnesium sulfate in aneurysmal subarachnoid hemorrhage : a randomized controlled trial. Stroke. 2005; 36:1011–1015. PMID: 15790946.

19. van den Bergh WM, Dijkhuizen RM, Rinkel GJ. Potentials of magnesium treatment in subarachnoid haemorrhage. Magnes Res. 2004; 17:301–313. PMID: 15726906.
20. Veyna RS, Seyfried D, Burke DG, Zimmerman C, Mlynarek M, Nichols V, et al. Magnesium sulfate therapy after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002; 96:510–514. PMID: 11883835.

21. Westermaier T, Stetter C, Vince GH, Pham M, Tejon JP, Eriskat J, et al. Prophylactic intravenous magnesium sulfate for treatment of aneurysmal subarachnoid hemorrhage : a randomized, placebo-controlled, clinical study. Crit Care Med. 2010; 38:1284–1290. PMID: 20228677.

22. Wong GK, Poon WS, Chan MT, Boet R, Gin T, Ng SC, et al. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (IMASH) : a randomized, double-blinded, placebo-controlled, multicenter phase III trial. Stroke. 2010; 41:921–926. PMID: 20378868.

23. Wong GK, Poon WS, Chan MT, Boet R, Gin T, Ng SC, et al. Plasma magnesium concentrations and clinical outcomes in aneurysmal subarachnoid hemorrhage patients : post hoc analysis of intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage trial. Stroke. 2010; 41:1841–1844. PMID: 20538692.

24. Yahia AM, Kirmani JF, Qureshi AI, Guterman LR, Hopkins LN. The safety and feasibility of continuous intravenous magnesium sulfate for prevention of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2005; 3:16–23. PMID: 16159090.
Fig. 2
Repeated serum concentrations of magnesium between the patients with symptomatic vasospasm and without vasospasm who had magnesium infusion. There was no difference in the serum concentrations of magnesium between the two groups (p=0.50).
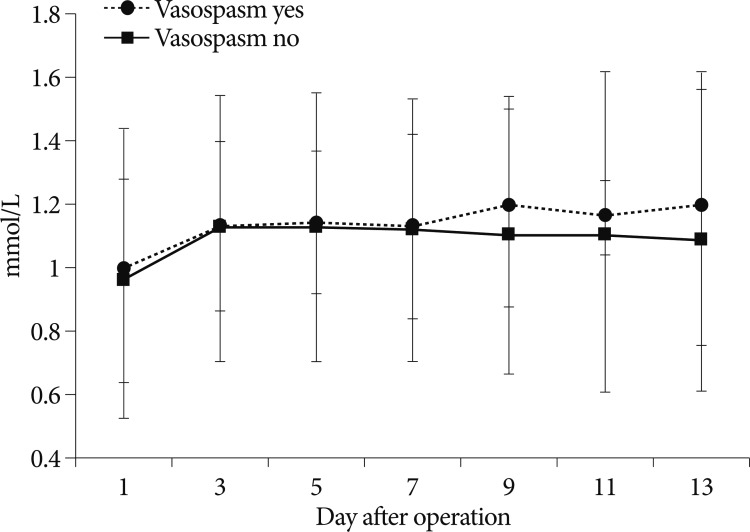
Table 2
Demographic and clinical characteristics of patients before propensity score matching in 157 patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download