Abstract
Objective
Hemifacial spasm (HFS) caused by vertebrobasilar dolichoectasia (VBD) is very rare, and in theses cases, it is difficult to decompress the nerve from its vascular compression. The objective of this study was to investigate the outcome of microvascular decompression (MVD) for HFS caused by VBD.
Methods
There were 10 patients of HFS caused by VBD at our hospital between September 1978 and September 2008. We evaluated magnetic resonance angiography (MRA) and time of flight magnetic resonance imaginge (TOF MRI) findings using the criteria for VBD. We compared the clinical outcomes of MVD for the 10 patients with VBD with the overall outcomes of the total 2058 MVDs performed for HFS.
Results
The results of MVD for HFS caused by VBD were successful in 90.9% of cases. The postoperative complication rate in VBD was 45.5%. Offending vessels in patients with VBD were identified visually during surgery. Adverse effects after MVD were found in 4 patients. We found that the diameter of VBD was significantly greater in patients with complications than in those with no complications (p=0.028).
Conclusion
Our data shows that MVD may be a good treatment modality for HFS caused by VBD but care must be taken to avoid adverse effects from the procedure. It is important to detach the dolichoectatic artery from its surrounding structures sufficiently to allow it to be easily movable. In addition, attempts should be made to lessen the retraction of the cerebellum during release of the dolichoectatic artery.
Hemifacial spasm (HFS) is a hyperactive cranial nerve dysfunction syndrome characterized by unilateral involuntary contractions of muscles innervated by the affected facial nerve. Causes of HFS include blood vessel, tumor, and bony abnormality15). Since Campbell and Keedy2) first described vascular arterial compression of the facial nerve among patients with HFS. The cause of this condition is thought to be mainly neurovascular compression of the facial nerve at its root exit zone (REZ) from the brainstem13). Therefore, microsurgical neurovascular decompression (MVD) is now widely performed as the most logical and curative treatment of HFS.
In general, the offending vessels are the anterior inferior cerebellar artery (AICA), posterior inferior cerebellar artery (PICA), vertebral artery (VA), or branches of these main arteries encountered during MVD. HFS caused by vertebrobasilar dolichoectasia (VBD), however, is quite rare30). The offending in cases of VBD is difficult to move during MVD due to its character of enlargement and elongation. We have experienced several cases of MVD for HFS resulting from VBD. We compared outcomes of MVD for HFS caused by VBD with the outcomes of non-VBD cases, and analyzed the complication occurrence rate in MVD for HFS related with VBD.
VBD refers to enlargement and elongation of the vertebrobasilar artery. To diagnose VBD, we need criteria that specifically define enlargement and elongation of the vertebrobasilar artery. Smoker et al.28,29) reviewed normal high-resolution computed tomographic (CT) scans of 123 patients and defined the diameter, the height of the bifurcation, and the transverse position of the normal basilar artery. According to Smoker's criteria, we use the term elongation if the basilar artery, at any point throughout its course, lies in a lateral position to the margin of the clivus or dorsum sellae (Position 2, 3) (Table 1) or if the artery bifurcation lies above the plane of the suprasellar cistern (Height 2, 3) (Table 2). Enlargement is diagnosed if the diameter of the basilar artery is greater than 4.5 mm. Giang et al.9) reported that MRI diagnosed VBD as well as CT, and it was proved to be superior to CT in delineating the anatomical relationship of the vessels to the neural structures. Therefore, although Smoker et al. defined the normal basilar artery based on data from CT imaging, we measured elongation and enlargement on time of flight magnetic resonance image (TOF MRI) and magnetic resonance angiography (MRA).
A total of 2,064 MVDs for HFS were performed at our institution between September 1978 and September 2008. In six patients, HFS was not resulting from a blood vessel, and these patients were excluded from the current study. Among the remaining 2058 cases with vascular compression, 2047 cases (99.5%) had non-VBD HFS, and 11 cases (0.5%) had HFS caused by VBD. One patient with HFS caused by VBD had bilateral HFS and underwent 2-stage MVD. The clinical outcomes and image findings of TOF MRI and MRA of these 11 cases were analyzed.
We retrospectively evaluated the available preoperative images from TOF MRI and MRA, which were performed with a 1.5-Tesla imaging system (Signa; GE Medical Systems, Milwaukee, WI, USA). Enlargement of the vertebrobasilar artery was measured on MRA by Picture Archiving Communication System (PACS; GE Medical Systems, Milwaukee, WI, USA) and diagnosed if the diameter of the basilar artery was greater than 4.5 mm. Elongation was evaluated on TOF MRI and diagnosed in the case of position 2 or 3 or height 2 or 3 (Table 1, 2).
In all patients, exposure and dissection were performed using standard surgical techniques for microvascular decompression procedures with the lateral decubitus position through a lateral retrosigmoid suboccipital craniectomy14,19).
In patients with VBD, a wider retrosigmoid suboccipital craniectomy was done to reach at the sigmoid sinus. Dissection of the arachnoid membranes was carefully continued from the lower cranial nerves up to the petrosal vein to enhance the exposure and mobilization of the cerebellum. Release of the VBD artery (VA or BA) was meticulously obtained not only near the REZ but also from the proximal portion to the distal for as great a distance as possible. In this way, the VBD artery was made to move easily, to allow complete decompression of the nerve, and stretching of the seventh and eighth cranial nerves was lessened during the insertion of Teflon felt and balls to reposition the offending vessels. After the vessel was freed, Teflon felt and balls were inserted in a step-by-step manner without retraction of the vessel. The first Teflon was placed between the vessel and the nerve beside the compression site and was then gently pushed along the nerve toward the compression site. As second Teflon was placed where the first was originally positioned, and this was repeated, until complete vascular decompression was achieved.
The clinical data and intra-operative findings of the selected patients were reviewed retrospectively. After MVD, the patients were classified into the following five grades, on the basis of the degree of HFS present : 1) "excellent" if no HFS was present; 2) "good" if the HFS was more than 90% resolved; 3) "fair" if the HFS was more than 50% resolved; 4) "poor" if the HFS was less than 50% resolved; and 5) "failure" for all remaining results. Outcomes of "excellent" and "good" were considered successful outcomes. Clinical outcomes were evaluated immediately after the surgery and at outpatient visits during the subsequent follow-up period.
Comparisons between two groups (non-VBD vs. VBD) were tested for statistical significance with the Fisher exact test. The complication occurrences of VBD were assessed using the Mann-Whitney test. Probability values were 2-tailed, and values of p<0.05 were considered significant. Case no 3 (Table 4) was excluded from statistical analysis, because it did not involve a dolichoectatic artery. All data analyses were performed with PASW statistics version 18.0 (SPSS Inc., Somers, NY, USA).
The clinical characteristics of 2,058 cases of HFS treated by MVD are summarized in Table 3. The diameter, height, and transverse position of the basilar arteries of the VBD cases are presented in Table 4. The mean diameter of the basilar arteries was 5.05 mm (range, 4.61-5.93 mm). Offending vessels identified visually during surgery were 4 AICA, 1 PICA, 1 VA, and 5 multiple artery. A patient with right offending VA (case no 4) (Fig. 1) had left-sided facial spasm. In all cases except one, the VA or basilar artery (BA, case no 2) (Fig. 2) affected neurovascular compression of the facial nerve directly or over the offending vessel. One case (case no 3) (Fig. 3) had only the AICA as the offending vessel, not related with VA or BA compression. This patient had bilateral HFS that required 2-stage MVD.
Levels of successful outcomes were 93.1% in non-VBD and 90.9% in VBD, a difference which was not statistically significant (p=0.779) (Table 5). Postoperative complications related to facial nerve and cochlear nerve in non-VBD were noted in 24.7% (permanent in 2.4%) these complications included facial palsy in 18.5% (permanent in 0.9%) and hearing impairment in 6.1% (permanent in 1.5%). However, the postoperative complication rate in VBD was 45.5%, greater than the rate of 24.7% in non-VBD. There was transient facial palsy in three cases (27.2%), transient hearing impairment in one case (9.1%), and one permanent case of facial palsy (9.1%). There was no statistical difference in the overall complication rate (p=0.154).
There were 5 cases of complications in the group of patients with VBD. Table 6 shows the results of the analysis of complication occurrence in VBD. There was no statistically significant difference in age and sex ratio between the complication and no complication groups. Diameter of the VBD was significantly greater in the complication group than in the group with no complications (p=0.028). However, there were no statistically significant differences in height and position of the VBD between the complication and no complication groups.
VBD is a rare arteriopathy characterized by elongation and enlargement of the vertebrobasilar artery with subsequent thrombosis, micro-embolization, and brainstem compression, with or without aneurysm formation6,11,12,16,18,21,31,34,35). This arteriopathy is known to cause variable neurologic deficits, including combined brainstem and cranial nerve syndromes3,6,7,12,24,31), cervicomedullary junction compression12,16,18,20,31,33) transient or permanent motor deficits12,16,20,21,23,35), cerebellar dysfunction16), central sleep apnea22), hydrocephalus, ischemic stroke33,35), and subarachnoid hemorrhage5,8,26). VBD is a potentially serious condition that may cause severe disability due to ischemic or compressive dysfunction in the posterior fossa27,32). VBD itself may cause hemodynamic changes leading to thrombosis and microembolization32). Furthermore, manipulation of the dolichoectatic artery during surgical procedures may also result in these hemodynamic changes. Therefore, an effort should be made to minimize the manipulation of the dolichoectatic artery in order to prevent ischemic dysfunction in the posterior fossa. In the procedure described here, the VBD artery was released from the proximal portion to the distal as far along its length as possible before decompression of the seventh nerve, and Teflon felt and balls were inserted in stepwise manner without retraction of the vessel. In our study, 10 patients had hemifacial spasm caused by VBD without any other neurologic deficits preoperatively, and fortunately, no ischemic dysfunction occurred following microsurgical neurovascular decompression.
Hemifacial spasm caused by VBD has been reported previously1,3,10,25,27,30). In these cases, vascular compression of the facial nerve at the root entry zone was considered the cause of HFS. There are also reported cases of HFS related with a dolichoectatic artery. Chakravarty4) reported HFS resulting from pontine compression by a large fusiform dolichoectatic basilar artery without any compression of the facial nerve at the REZ. In all our cases, we identified an offending artery compressing the facial nerve at the REZ. There was direct compression of the facial nerve by the VA in six cases, including right VA compression of the left facial nerve (Fig. 1), compression by the VA or BA (Fig. 2) over the offending vessel in four cases, and compression by the AICA alone, with no relation to a dolichoectatic artery (Fig. 3), in one case. One patient (case no 1 and 3) (Fig. 3) who had only the AICA offending vessel on the right side, underwent microsurgical neurovascular decompression for HFS in left side first. The left side offending vessels were the VA and PICA. Even though this patient had VBD, microsurgical neurovascular decompression for the right side was similar to other usual decompression procedure. A dolichoectatic artery as the offending vessel or over the offending vessel makes surgical procedures difficult to perform completely without any complications because of its character of enlargement and elongation.
The prevalence of VBD is 0.5% of all HFS patients in this study. Even though VBD is a very rare arteriopathy, there is the problem of selection and referral bias in these results. The male to female ratio in VBD is greater than in non-VBD (1 : 1.2 vs. 1 : 3). Multiple offending vessels are more frequent in VBD than in non-VBD (45.5% vs. 12.5%). In our study, a success rate of 90.9% was achieved in VBD, similar to the 93.2% success rate in non-VBD (p=0.779). If the dolichoectatic artery is released only near the REZ, it is difficult and dangerous to retract the dolichoectatic artery to directly insert Teflon felt and balls into the compression site because of the artery's character of enlargement and the ischemic attack risk. Successful neurovascular decompression could be performed without retraction of dolichoectatic artery with sufficient release of the artery and a step-by-step manner of insertion of the Teflon. It is thus a mandatory step in neurovascular decompression to release the dolichoectatic artery from surrounding structures enough to allow its easy movement, as in the procedure described here. Nevertheless, one patient in our study (case no 1) developed transient facial weakness and hearing impairment, two patients (case no 2, 6) developed transient facial weakness, and one (case no 4) developed permanent facial weakness. Although the difference was not statistically significant (p=0.154), the postoperative complication rate (45.5%) in VBD was higher than that in non-VBD (24.7%). Excessive retraction of the cerebellar flocculus to obtain surgical visibility during release of the dolichoectatic artery from its surrounding structure might play a role in increasing the complication rate. We analyzed the occurrence of complications in VBD (Table 6). Our findings suggest that the degree of height and position of the VBD may not contribute to the occurrence of complications. Only increase of diameter of the VBD was significantly associated with the occurrence of complications (p=0.028) in our study. This result shows that the enlargement character of the VBD may be meaningful in development of complications. In fact, increase of a dolichoectatic artery's diameter results in much more increase of volume of that artery. Therefore, much greater retraction of the cerebellum may be needed to obtain sufficient release of the artery. A wider craniectomy and dissection of the arachnoid membranes from the lower cranial nerves up to the petrosal vein to enhance the exposure and mobilization of the cerebellum, the procedures followed here, are helpful for lessening retraction of cerebellum during release of the dolichoectatic artery, but not enough to prevent complications. Recently, some authors have used an endoscope to assist in the decompression procedure of trigeminal neuralgia or HFS caused by an elongated, tortuous or enlarged VA or BA17). The use of an endoscope is another method that may prevent complications through lessening retraction of the cerebellum. To prevent postoperative complications, minimal manipulation of the dolichoectatic artery and lessening retraction of the cerebellum should be achieved.
In this study, there were no statistically significant difference in surgical outcomes of MVD between VBD and non-VBD. Therefore, we would recommend microsurgical neurovascular decompression as treatment for hemifacial spasm caused by VBD. To obtain good outcomes and to reduce postoperative complication, the dolichoectatic artery, from the proximal portion to the distal, must be made sufficiently free from its surrounding structures before neurovascular decompression without excessive retraction of the cerebellum.
Acknowledgements
This study was supported by the Industrial Source Technology Department Program (No. 10033812) of the Ministry of Knowledge Economy (MKE) of Korea.
References
1. Arantes M, Garcia R, Pereira JR, Costa M. [Hemifacial spasms and vertebral dolichoectasias]. Rev Neurol. 2008; 47:468–470. PMID: 18985596.
2. Campbell E, Keedy C. Hemifacial spasm; a note on the etiology in two cases. J Neurosurg. 1947; 4:342–347. PMID: 20254516.

3. Carella A, Caruso G, Lamberti P. Hemifacial spasm due to elongation and ectasia of the distal segment of the vertebral artery. Report of two cases. Neuroradiology. 1973; 6:233–236. PMID: 4772449.

4. Chakravarty A. Hemifacial spasm, glossodynia, and dolichoectasia of the basilar artery. J Stroke Cerebrovasc Dis. 2012; 21:78–81. PMID: 20833080.

5. De Georgia M, Belden J, Pao L, Pessin M, Kwan E, Caplan L. Thrombus in vertebrobasilar dolichoectatic artery treated with intravenous urokinase. Cerebrovasc Dis. 1999; 9:28–33. PMID: 9873160.

6. de Oliveira Rde M, Cardeal JO, Lima JG. [Basilar ectasia and stroke : clinical aspects of 21 cases]. Arq Neuropsiquiatr. 1997; 55:558–562. PMID: 9629405.
7. Deeb ZL, Jannetta PJ, Rosenbaum AE, Kerber CW, Drayer BP. Tortuous vertebrobasilar arteries causing cranial nerve syndromes : screening by computed tomography. J Comput Assist Tomogr. 1979; 3:774–778. PMID: 229135.
8. Flemming KD, Josephs K, Wijdicks EF. Enlarging vertebrobasilar dolichoectasia with subarachnoid hemorrhage heralded by recurrent ischemia. Case illustration. J Neurosurg. 2000; 92:504. PMID: 10701548.

9. Giang DW, Perlin SJ, Monajati A, Kido DJ, Hollander J. Vertebrobasilar dolichoectasia : assessment using MR. Neuroradiology. 1988; 30:518–523. PMID: 3226539.
10. Han IB, Chang JH, Chang JW, Huh R, Chung SS. Unusual causes and presentations of hemifacial spasm. Neurosurgery. 2009; 65:130–137. discussion 137. PMID: 19574834.

11. Himi T, Kataura A, Tokuda S, Sumi Y, Kamiyama K, Shitamichi M. Downbeat nystagmus with compression of the medulla oblongata by the dolichoectatic vertebral arteries. Am J Otol. 1995; 16:377–381. PMID: 8588634.
12. Hongo K, Nakagawa H, Morota N, Isobe M. Vascular compression of the medulla oblongata by the vertebral artery : report of two cases. Neurosurgery. 1999; 45:907–910. PMID: 10515488.

13. Ishikawa M, Ohira T, Namiki J, Kobayashi M, Takase M, Kawase T, et al. Electrophysiological investigation of hemifacial spasm after microvascular decompression : F waves of the facial muscles, blink reflexes, and abnormal muscle responses. J Neurosurg. 1997; 86:654–661. PMID: 9120630.

14. Jannetta PJ, Abbasy M, Maroon JC, Ramos FM, Albin MS. Etiology and definitive microsurgical treatment of hemifacial spasm. Operative techniques and results in 47 patients. J Neurosurg. 1977; 47:321–328. PMID: 894338.
15. Kim JP, Park BJ, Choi SK, Rhee BA, Lim YJ. Microvascular decompression for hemifacial spasm associated with vertebrobasilar artery. J Korean Neurosurg Soc. 2008; 44:131–135. PMID: 19096662.

16. Kim P, Ishijima B, Takahashi H, Shimizu H, Yokochi M. Hemiparesis caused by vertebral artery compression of the medulla oblongata. Case report. J Neurosurg. 1985; 62:425–429. PMID: 3973710.

17. Lin CF, Chen HH, Hernesniemi J, Lee CC, Liao CH, Chen SC, et al. An easy adjustable method of ectatic vertebrobasilar artery transposition for microvascular decompression. Clin Neurol Neurosurg. 2012; 114:951–956. PMID: 22390889.

18. Maruyama K, Tanaka M, Ikeda S, Tada T, Yanagisawa N. [A case report of quadriparesis due to compression of the medulla oblongata by the elongated left vertebral artery]. Rinsho Shinkeigaku. 1989; 29:108–111. PMID: 2743680.
19. McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves : lessons learned after 4400 operations. J Neurosurg. 1999; 90:1–8. PMID: 10413149.

20. Milandre L, Bonnefoi B, Pestre P, Pellissier JF, Grisoli F, Khalil R. [Vertebrobasilar arterial dolichoectasia. Complications and prognosis]. Rev Neurol (Paris). 1991; 147:714–722. PMID: 1775825.
21. Milandre L, Martini P, Perot S, Mercier C. Pure motor hemiparesis in a case of vertebrobasilar arterial ectasia. Neuroradiology. 1993; 35:196–198. PMID: 8459918.

22. Miyazaki M, Hashimoto T, Sakurama N, Yoshimoto T, Tayama M, Kuroda Y. Central sleep apnea and arterial compression of the medulla. Ann Neurol. 1991; 29:564–565. PMID: 1859186.

23. Moss MS, West RJ. Ectasia of the vertebral artery as a cause of isolated pyramidal tract signs : case report and a review of the literature. Br J Neurosurg. 1996; 10:497–499. PMID: 8922711.
24. Nuti D, Passero S, Di Girolamo S. Bilateral vestibular loss in vertebrobasilar dolichoectasia. J Vestib Res. 1996; 6:85–91. PMID: 8925119.

25. Papapetropoulos S, Argyriou AA, Guevara A, Sengun C, Mitsi G, Singer C. Hemifacial spasm and pontine compression caused by a giant vertebrobasilar dolichoectasia. Cerebrovasc Dis. 2009; 27:413–414. PMID: 19295203.

26. Rabb CH, Barnwell SL. Catastrophic subarachnoid hemorrhage resulting from ruptured vertebrobasilar dolichoectasia : case report. Neurosurgery. 1998; 42:379–382. PMID: 9482190.

27. Rahman EA, Trobe JD, Gebarski SS. Hemifacial spasm caused by vertebral artery dolichoectasia. Am J Ophthalmol. 2002; 133:854–856. PMID: 12036693.

28. Smoker WR, Corbett JJ, Gentry LR, Keyes WD, Price MJ, McKusker S. High-resolution computed tomography of the basilar artery : 2. Vertebrobasilar dolichoectasia : clinical-pathologic correlation and review. AJNR Am J Neuroradiol. 1986; 7:61–72. PMID: 3082145.
29. Smoker WR, Price MJ, Keyes WD, Corbett JJ, Gentry LR. High-resolution computed tomography of the basilar artery : 1. Normal size and position. AJNR Am J Neuroradiol. 1986; 7:55–60. PMID: 3082144.
30. Titlić M, Vrebalov-Cindro V, Lahman-Dorić M, Buca A, Jukić I, Tonkić A. Hemifacial spasm in vertebrobasilar dolichoectasia. Acta Neurol Belg. 2006; 106:23–25. PMID: 16776433.
31. Ubogu EE, Chase CM, Verrees MA, Metzger AK, Zaidat OO. Cervicomedullary junction compression caused by vertebral artery dolichoectasia and requiring surgical treatment. Case report. J Neurosurg. 2002; 96:140–143. PMID: 11794596.

32. Ubogu EE, Zaidat OO. Vertebrobasilar dolichoectasia diagnosed by magnetic resonance angiography and risk of stroke and death : a cohort study. J Neurol Neurosurg Psychiatry. 2004; 75:22–26. PMID: 14707300.
33. Vincentelli F, Caruso G, Rabehanta PB, Rey M. Surgical treatment of a rare congenital anomaly of the vertebral artery : case report and review of the literature. Neurosurgery. 1991; 28:416–420. PMID: 2011224.

34. Wolfe T, Ubogu EE, Fernandes-Filho JA, Zaidat OO. Predictors of clinical outcome and mortality in vertebrobasilar dolichoectasia diagnosed by magnetic resonance angiography. J Stroke Cerebrovasc Dis. 2008; 17:388–393. PMID: 18984433.

35. Yu YL, Moseley IF, Pullicino P, McDonald WI. The clinical picture of ectasia of the intracerebral arteries. J Neurol Neurosurg Psychiatry. 1982; 45:29–36. PMID: 7062068.

Fig. 1
Preoperative MRI and photograph taken during surgery of a patient with right VA offender. The patient was 69-year-old man with a left side facial spasm for 5 years. Preoperative MRI shows that right side VA compressed left facial nerve at the REZ (red arrows). Photograph was taken before neurovascular decompression was performed. Surgical outcome was 'good' but permanent facial palsy developed. VA basilar artery, VII facial nerve. VA : vertebral artery, REZ : root exit zone.

Fig. 2
Preoperative MRI of a patient with AICA offender and BA over AICA. The patient was 48-year-old man with right side facial spasm for 2 years. Preoperative MRI shows that AICA compressed facial nerve and BA is over AICA offender (circle) at the REZ. During MVD, we made an effort to make BA movable freely. After surgery, HFS completely resolved but transient facial palsy occurred. AICA : anterior inferior cerebellar artery, REZ : root exit zone, MVD : microsurgical neurovascular decompression, BA : basilar artery.
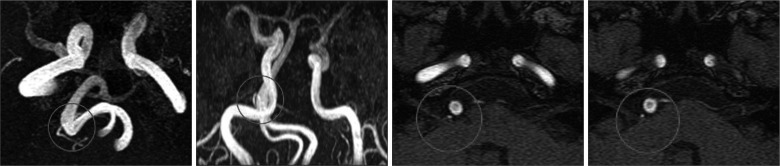
Fig. 3
Preoperative and postoperative MRI of a patient with bilateral HFS. The patient was 50s woman with bilateral HFS. Left side facial spasm resulting from VA and PICA occurred one year before first MVD. Preoperative MRI shows that dolichoectatic VA and PICA compressing left facial nerve at the REZ (short arrow). After MVD, left HFS completely resolved but transient facial palsy and hearing impairment occurred. Right side HFS occurred 4 years after left MVD. We obtained postoperative MRI showing that only AICA compressed facial nerve (long arrow) and that complete decompression of left facial nerve was done (circle). After right MVD, right HFS completely disappeared without any complication. In the right HFS, VBD did not affect the offender. HFS : hemifacial spasm, VA : vertebral artery, PICA : posterior inferior cerebellar artery, REZ : root exit zone, MVD : microsurgical neurovascular decompression, VBD : vertebrobasilar dolichoectasia.
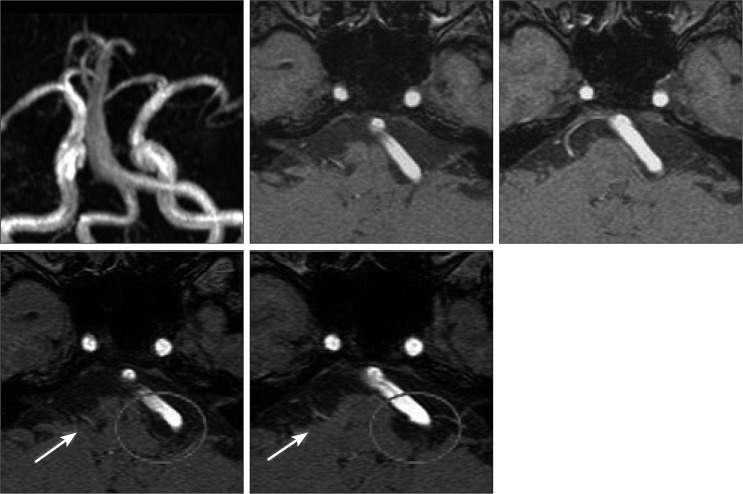
Table 3
Clinical characteristics of 2058 cases with hemifacial spasm treated by microvascular decompression

Table 4
Summary of eleven cases of hemifacial spasm caused by vertebrobasilar dolichoectasia
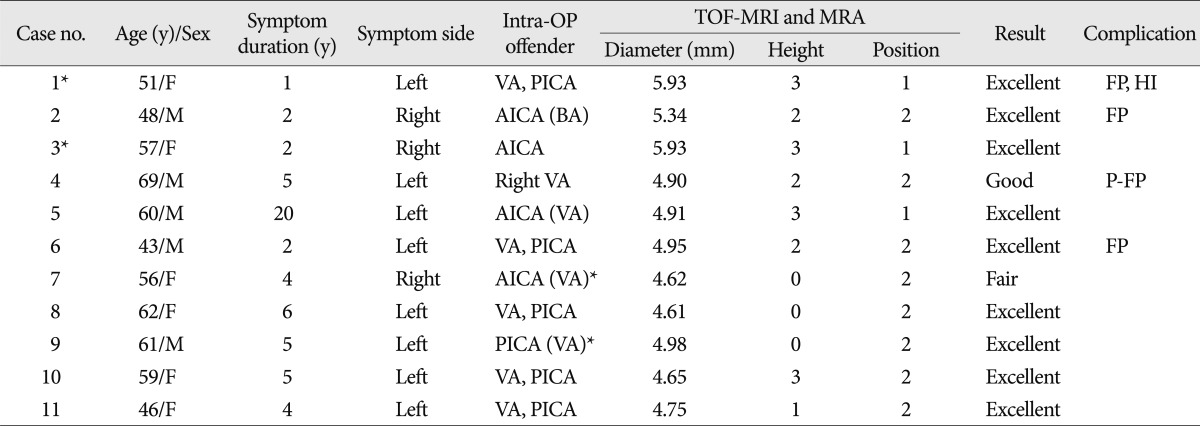
*one patient who had bilateral HFS and underwent MVD twice. ( ) : over offender vessel, not direct contact. TOF-MRI : time of flight magnetic resonance image, VA : vertebral artery, PICA : posterior inferior cerebellar artery, AICA : anterior inferior cerebellar artery, BA : basilar artery, FP : facial palsy. P-FP : permanent facial palsy, HI : hearing impairment




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download