Abstract
Eosinophilic myelitis (EM) or atopic myelitis is a rare disease characterized by a myelitic condition in the spinal cord combined with allergic process. This disease has specific features of elevated serum IgE level, active reaction to mite specific antigen and stepwise progression of mostly the sensory symptoms. Toxocariasis can be related with a form of EM. This report describes two cases of cervical eosinophilic myelitis initially considered as intramedullary tumors. When a differential diagnosis of the intramedullary spinal cord lesion is in doubt, evaluation for eosinophilic myelitis and toxocariasis would be beneficial.
Differential diagnosis of various intramedullary cord lesions is sometimes very difficult10). Discernment of the intramedullary tumor is of special concern to neurosurgeons, and if a surgeon has simple diagnostic tools to rule out intramedullary cord tumor, invasive and destructive tissue confirmation can be avoided.
Allergic reaction might be related to a myelitic condition, which is called eosinophilic myelitis (EM) or atopic myelitis. Although this is a rare disease unfamiliar to neurosurgeons, laboratory findings such as hyperIgEaemia and positivity to mite antigen-specific IgE are well known1,5,7,8,11). Perivascular lymphocyte cuffing, disrupted small vessels, and especially eosinophilic infiltration in the spinal cord in the neuropathological study supports the association of allergy and the myelitic condition. This disease can easily be misdiagnosed as transverse myelitis, autoimmune disease or even intramedullary gliotic tumor by image findings6,12). However, with the aid of laboratorial studies and clinical features, EM can be briefly confirmed. Recently, association of toxocariasis with EM was reported, and antihelminthic drugs are being focused as an effective treatment.
This report describes two cases of eosinophilic myelitis associated with toxocariasis with their laboratorial, clinical and characteristic radiological features.
A 71-year-old man presented with a neck pain and progressing hypesthesia in the left arm and leg for a month. He had a lumbar spine surgery for spinal stenosis three years prior to the visit. The neurologic examination revealed hyperesthesia in the left side and decreased proprioception below C7 dermatome, but no definite motor weakness or pathologic reflex was observed. The initial cervical magnetic resonance imaging (MRI) study showed an intramedullary high signal intensity (HSI) lesion on T2-weighted image (WI) in the C3-6 spinal cord without swelling, and mild enhancement in the dorsal column at the C5 level (Fig. 1). Motor evoked potential (MEP) was normal while somatosensory evoked potential (SEP) showed increased latency and the wave attenuation. In the laboratorial study, slightly elevated eosinophil count (5.9%) was observed, anti-nuclear antibody (Ab) was positive, and paraneoplastic Ab was negative. In the cerebrospinal fluid (CSF) study, cell count was clear and IgG level in CSF was elevated (10.46 mg/dL; normal range 0.63-3.35), whereas oligoclonal band was negative. With the impression of myelitis of unknown etiology, steroid was administered. Although the patient being neurologically stable, a follow up MRI after 3 months revealed aggravation of HSI lesion with more conspicuous nodular enhancement in the dorsal column at C5 level (Fig. 1). In suspicion of anaplastic intramedullary gliotic tumor, a cord biopsy was performed, targeting dorsally enhanced portion of the spinal cord. A midline myelotomy was done and a friable and grayish lesion was found. SEP/MEP were monitored during the procedure and when SEP wave attenuation was observed in the left side, the biopsy stopped. The patient reported aggravation of hypesthesia in the left leg by 30% after operation.
Infiltration of chronic, mixed inflammatory cells such as lymphocytes, histiocytes, eosinophils and plasma cells were noted in the pathological study. Immunohistochemical staining of glial fibrillary acidic protein and neurofilament showed marked disruption of neuroglial tissue. These findings led to the diagnosis of eosinophilic myelitis and vasculitis (Fig. 2).
Evaluation for allergy was performed after surgery. Total serum IgE was elevated, the allergic reaction for Dermatophagoides farinae (D. farinae) and IgG for toxocariasis was positive in the serum study. The neurological status of the patient was stable without further progression of motor weakness for 24 months with intermittent steroid therapy and albendazole medication.
A 55-year-old man was referred from local hospital due to tingling sensation in the left upper extremities and having radiological abnormalities. The original impression was intramedullary astrocytoma. The patient's motor power was intact, and pathological reflexes were not found. The outside MRI showed HSI lesion with the cord swelling at the level of 4-6th cervical spine and a focal nodular enhancement confined to dorsal segment at C5 (Fig. 3). The SEP study described increased latency and wave attenuation, whereas the MEP was normal. CSF findings were within the normal range, and no evidence of autoimmune disease was found. Similar to case 1, total IgE was elevated and the response to D. farinae was active in the serum study. IgG for Toxocariasis antigen was also positive. With the impression of eosinophilic myelitis by laboratory, radiological and clinical findings, steroid and albendazole were administered. Three months later, the cord HSI lesion was markedly improved in the follow-up MRI (Fig. 3). The clinical symptom was improved and stable for 8 months.
EM or atopic myelitis is defined as a localized myelitis in patients with hyperIgEaemia and mite antigen specific IgE. In spite of the neuropathological features being well described, the exact pathogenesis and clinical features of this disease are not fully understood. Parasitic infection, fungal infection, sarcoidosis, and Churg-Strauss syndrome are thought to be associated with EM. Particularly in Korea, EM patients reported to be positive to Toxocara excretory-secretary (TES) antibody in 93.9%7). This is comparable to the fact that seroprevalence of toxocariasis in rural Korean adults was approximately 5%. Also, specific IgE to D. farinae was reported to be detected in 100% of the Korean patients7). Characteristic eosinophil infiltration has rarely been encountered in other kind of myelitis and may be a proof of the relation between EM and allergic mechanism.
EM is usually diagnosed by clinical and serologic features, and its pathological confirmation through biopsy is not always compulsory, as in this case study. Several clinical features are known in studies with Japanese patients. 1) stepwise progression and fluctuation of the clinical course, 2) sensory dysfunction as the main symptoms and relatively infrequent motor weakness, 3) myelitic lesion on MRI, 4) Increased IgG in serum and mite antigen-specific IgG positivity, and 5) mild eosinophilia1,5,9,11). It is important to make a differential diagnosis of various neurological disorders of the intramedullary lesions. In transverse myelitis, it is usually hard to find the pathogenic factors, and differentiation from intramedullary cord tumor is the main concern to neurosurgeons. When diagnostic confirmation is difficult, physicians usually perform CSF study, various laboratorial tests, or delayed MRI. If intramedullary cord tumor is still suspected, invasive and destructive tissue biopsy may be considered. EM might be considered if stepwise progression or fluctuation of primarily sensory symptom is observed, and sufficient evaluation for allergy should be performed before invasive procedures. The aim of this report is to inform neurosurgeons on the diagnostic tools and clinical characteristics of EM and to avoid the risk of further neurological deterioration by unnecessary invasive spinal cord biopsy.
Various MRI findings are known, and HSI lesion in T2WI, and cord swelling with patchy enhancement are frequently found5,7,9). Enhancement of the lesion on post-contrast MRI results from focal blood-spinal barrier disruption due to reactive inflammatory process. Inflammation and infiltrated eosinophils may contribute to the anatomical and functional neural damage8,10). Unlike the previous reports, the enhancement was confined to the posterior column in our cases. This finding may explain the restriction of the symptoms to sensory function.
Accumulation of patients' data with EM and their MRI findings should be performed to characterize the enhancing pattern exactly.
EM is clearly related to the allergic mechanism such as elevated serum IgE level and mite antigen-specific IgE positivity. Myelitis with toxocariasis has rarely been reported, even though toxocariasis is a worldwide-occurring parasitic infection. The characteristics of Toxocara myelitis are very similar to those of EM; mainly sensory symptoms, HSI lesion on T2WI MRI, increased IgE and eosinophilia and eosinophilic infiltration on tissue study2,4-6). Toxocariasis is caused by Toxocara canis or Toxocara cati in man via incidental consumption of larvae in raw meat or animal liver. The freely migrating larvae can induce immune-mediated hypersensitivity reactions like hyperIgEaemia6,7,11). If the larvae migrate to central nervous system, they can cause local inflammation with hypersensitivity reaction and neurological deteriorations3). As describe by Lee et al.7), many Korean males have a chance of eating raw animal tissue in their social activities. Although exact pathomechanism of the larvae in EM is not well understood, Toxocara myelitis might play a considerable role in provoking EM. A diagnosis of toxocariasis is made by detection of antibody (IgG) for TES-antigen using ELISA method, and this test has high reliability because serologic cross-reaction of toxocariasis is very low2,3,6,11). Therefore, we suggest that in case of uncertain myelitis or intramedullary tumorous condition, evaluation for toxocariasis should be done and this might be beneficial. This is the first report illustrating neuropathological findings of EM with proven toxocariasis.
EM has been known to have relatively fair prognosis, because the patient usually present with clinical symptom of stepwise progression without muscle weakness. Systemic steroid administration is known to be effective in controlling clinical symptoms and improving radiologic findings in some cases. In case of toxocara induced EM, antihelminthic drugs like albendazole were reported to be more efficient than conventional steroid therapy in improving clinical status7,11). The dose and duration of the administration is not determined.
References
1. Goffette S, Jeanjean AP, Duprez TP, Bigaignon G, Sindic CJ. Eosinophilic pleocytosis and myelitis related to Toxocara canis infection. Eur J Neurol. 2000; 7:703–706. PMID: 11136359.

2. Gregoire SM, Mormont E, Laloux P, Godfraind C, Gilliard C. Atopic myelitis : a clinical, biological, radiological and histopathological diagnosis. J Neurol Sci. 2006; 247:231–235. PMID: 16782129.

3. Kikuchi H, Osoegawa M, Ochi H, Murai H, Horiuchi I, Takahashi H, et al. Spinal cord lesions of myelitis with hyperIgEemia and mite antigen specific IgE (atopic myelitis) manifest eosinophilic inflammation. J Neurol Sci. 2001; 183:73–78. PMID: 11166798.

4. Kim YR, Song JH, Park HK, Kim SH. A retrospective analysis of MRI-verified 29 cases of transverse myelitis. J Korean Neurosurg Soc. 2000; 29:1642–1649.
5. Kira J, Kawano Y, Horiuchi I, Yamada T, Imayama S, Furue M, et al. Clinical, immunological and MRI features of myelitis with atopic dermatitis (atopic myelitis). J Neurol Sci. 1999; 162:56–61. PMID: 10064169.

6. Lee IH, Kim ST, Oh DK, Kim HJ, Kim KH, Jeon P, et al. MRI findings of spinal visceral larva migrans of Toxocara canis. Eur J Radiol. 2010; 75:236–240. PMID: 19447576.

7. Lee JY, Kim BJ, Lee SP, Jeung YJ, Oh MJ, Park MS, et al. Toxocariasis might be an important cause of atopic myelitis in Korea. J Korean Med Sci. 2009; 24:1024–1030. PMID: 19949655.

8. Na SJ, Lee KO, Ko JH. Eosinophilic vasculitis of the spinal cord associated with Churg-Strauss syndrome. J Neurol Sci. 2010; 295:107–109. PMID: 20538303.

9. Osoegawa M, Ochi H, Kikuchi H, Shirabe S, Nagashima T, Tsumoto T, et al. Eosinophilic myelitis associated with atopic diathesis : a combined neuroimaging and histopathological study. Acta Neuropathol. 2003; 105:289–295. PMID: 12557017.

10. Schmutzhard E. Eosinophilic myelitis, a souvenir from South East Asia. Pract Neurol. 2007; 7:48–51. PMID: 17430865.
11. Shon HS, Kim SH, Lee SH, Lee SY, Kim SE, Yeo MJ. Eosinophilic myelitis associated with toxocariasis. J Korean Neurol Assoc. 2009; 27:176–178.
12. Taylor MR, Keane CT, O'Connor P, Mulvihill E, Holland C. The expanded spectrum of toxocaral disease. Lancet. 1988; 1:692–695. PMID: 2895221.

Fig. 1
A : A T2 weighted magnetic resonance (MR) sagittal image shows a high signal intensity lesion and swelling in the cervical spinal cord at the level of C3-6. B : A follow-up MR after three months reveals an aggravated high signal intensity lesion and cord swelling in C2-7. C : Contrast-enhanced T1 axial image shows focal nodular enhancement on posterior segment of spinal cord at the level of C5.
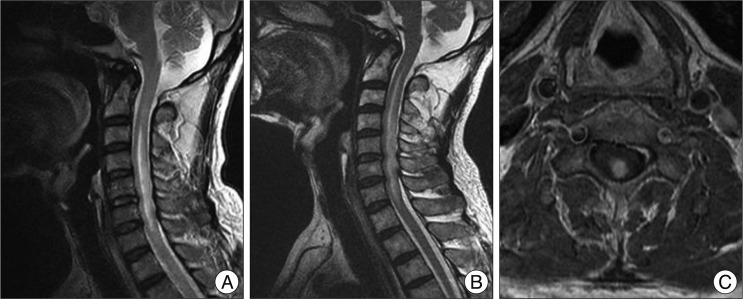
Fig. 2
Histologic findings of biopsy specimens from spinal cord, C5 level. Numerous infiltration of chronic, mixed inflammatory cells such as lymphocytes, histiocytes, and plasma cells are found diffusely on microscopic examination (×100, H&E) (A). Specially, infiltration of eosinophils is conspicuous (×400, H&E) (B). Immunohistochemical staining of glial fibrillary acidic protein (C) and neurofilament (D) shows disruption of neuroglial tissues suggesting inflammation.
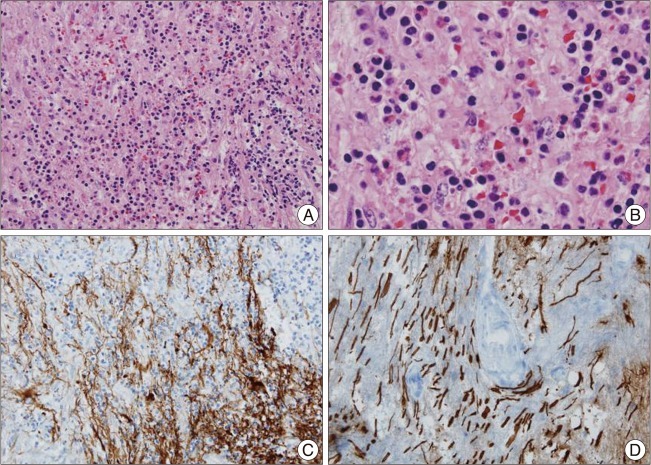
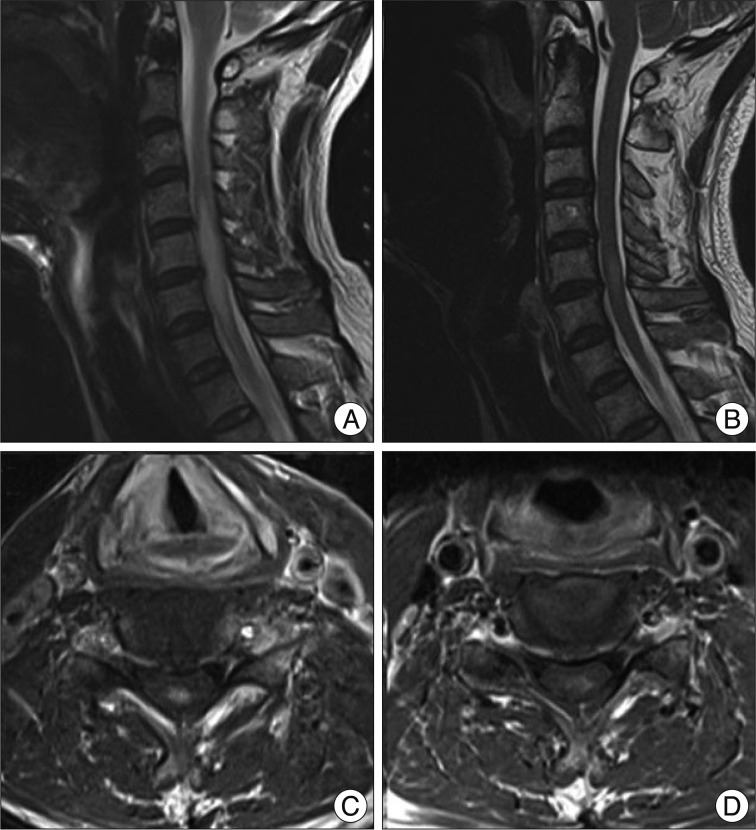
Fig. 3
A : A high signal intensity lesion and mild cord swelling was observed in C4-6 on T2 weight MRI. B : A nodular enhancement confined to posterior column was found at C5 level. C : After systemic steroid and albendazole medication, markedly decreased extent of HSI lesion and decreased swelling of spinal cord are observed after three months. D : The extent of nodular enhancing lesion is also decreased. MRI : magnetic resonance imaging, HSI : high signal intensity.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download