Abstract
Objective
The predictors of cranioplasty infection after decompressive craniectomy have not yet been fully characterized. The objective of the current study was to compare the long-term incidences of surgical site infection according to the graft material and cranioplasty timing after craniectomy, and to determine the associated factors of cranioplasty infection.
Methods
A retrospective cohort study was conducted to assess graft infection in patients who underwent cranioplasty after decompressive craniectomy between 2001 and 2011 at a single-center. From a total of 197 eligible patients, 131 patients undergoing 134 cranioplasties were assessed for event-free survival according to graft material and cranioplasty timing after craniectomy. Kaplan-Meier survival analysis and Cox regression methods were employed, with cranioplasty infection identified as the primary outcome. Secondary outcomes were also evaluated, including autogenous bone resorption, epidural hematoma, subdural hematoma and brain contusion.
Results
The median follow-up duration was 454 days (range 10 to 3900 days), during which 14 (10.7%) patients suffered cranioplasty infection. There was no significant difference between the two groups for event-free survival rate for cranioplasty infection with either a cryopreserved or artificial bone graft (p=0.074). Intergroup differences according to cranioplasty time after craniectomy were also not observed (p=0.083). Poor neurologic outcome at cranioplasty significantly affected the development of cranioplasty infection (hazard ratio 5.203, 95% CI 1.075 to 25.193, p=0.04).
Decompressive craniectomy is the standard surgical treatment for malignant cerebral edema and brain herniation resulting from cerebral infarction, intracranial hemorrhage and severe traumatic brain injury6,16,18,20,24). After decompressive craniectomy, cranioplasty is also generally performed for the purpose of cosmetic recovery and to protect against the development of the syndrome of the trephined5).
It is very important to minimize the complications of cranioplasty as a secondary surgery3,5). Infection of the cranioplasty site has been shown to elevate craniectomy-related morbidity and can lead to the long-term use of antibiotics or removal of the graft material and subsequent repeat cranioplasty4). After decompressive craniectomy, cranial defects are repaired with cryopreserved or artificial bone grafts at different time intervals. Although a recent systematic review assessed cranioplasty infection according to early surgery, material, and method of flap preservation23), the risk factors for cranioplasty infection remain to be elucidated.
The present study aimed to investigate the long-term incidence and associated factors of the cranioplasty infection according to graft materials and the time interval between craniectomy and cranial repair, along with other factors that may contribute to surgical site infection.
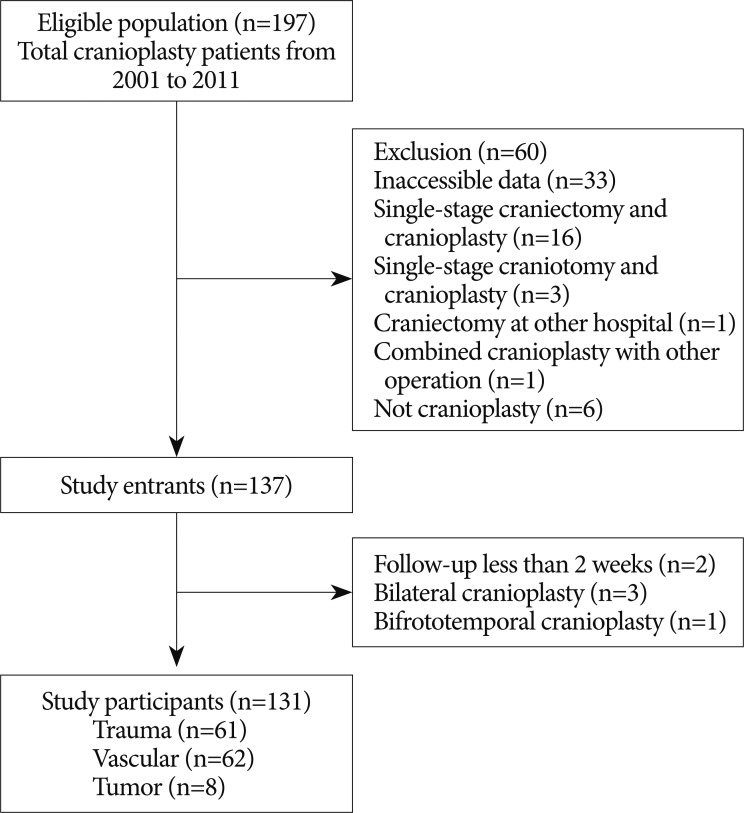
This single-center retrospective cohort study was designed to assess differences in the incidence of cranioplasty infection based on bone graft material and the time interval between craniectomy and cranioplasty and to examine other potential factors contributing to cranioplasty site infection. The inclusion criteria included adult patients who underwent decompressive craniectomy and subsequent cranioplasty between January 2001 and December 2011. Those who underwent craniectomy at another hospital but cranioplasty at our hospital were also included if the cause and time of craniectomy was identifiable. Exclusion criteria were as follows : 1) inaccessible patient data; 2) single-stage craniectomy and cranioplasty; 3) single-stage craniotomy and cranioplasty; 4) craniectomy at other hospital with insufficient data; 5) combined cranioplasty with other operation; 6) operation which was not a cranioplasty. Cases of cranioplasty infection were defined as those that needed further surgery for bone graft removal due to infection or those in which antibiotics were administered longer than 2 weeks after cranioplasty under the assumption of the presence of site infection. The eligible population included 197 cranioplasty patients during the target period. Among them, 60 patients were excluded, leaving a total of 137 patients (Fig. 1). The final number of participants was 131 due to 2 patients with less than 2 weeks of follow-up after cranioplasty, 3 patients with single-stage bilateral cranioplasty, and 1 patient with bifrontotemporal cranioplasty (Fig. 1). The study protocol was approved by the Institutional Review Board of Incheon St. Mary's Hosptial, the Catholic University of Korea (No. OC12RISI0025).
All autogenous bone flaps acquired from decompressive craniectomies were preserved in a deep freezer at a temperature of -71℃. During the cranioplasty, connective tissues such as pericranium, muscle, fascia and galea were entirely removed. Two sterilization methods were performed. From 2001 to 2008, ethylene oxide (EO) gas sterilization of cryopreserved bone material stored in a deep freezer was conducted, and tissues adhering to the bone graft were removed and irrigated with saline before the graft implantation. From 2009 to 2011, chemical sterilization of autogenous bone material was carried out, and blood clots or other tissues on the cryopreserved bone were cleaned with normal saline after soaking several times in hydrogen peroxide and alcohol alternatively for 30 minutes each. Prophylactic antibiotics were administered 30 minutes before the incision during the cranioplasty; other operative procedures varied according to neurosurgeons.
Patients were evaluated based on baseline characteristics including sex, age, medical history, cause of craniectomy, location of cranioplasty, graft materials, duration of cranioplasty (duration of general anesthesia), Glasgow outcome scale (GOS) at cranioplasty, type and duration of prescription prophylactic antibiotics, preservation method used for the autogenous bone flap and time interval between craniectomy and cranioplasty. Medical problems included diabetes, hypertension, cancer, allergies, thyroid disease, dyslipidemia and heart disease. The cause of decompressive craniectomy fell into three categories : trauma, vascular and tumor cases. The lateralization of the cranioplasty was classified as right, left or bilateral. The duration of use of prophylactic antibiotics was categorized as under 1 week or over 1 week. Graft material was classified as cryopreserved autogenous bone graft or artificial graft material. The time period from craniectomy to cranioplasty was calculated to the day, and patients were categorized into two groups of where early cranioplasty occurred within 3 months and late cranioplasty after 3 months.
The event as the primary outcome was defined as cranioplasty infection, and this condition was satisfied using any of the following criteria : 1) records showing a prescription for antibiotics more than 2 weeks after cranioplasty without other organ infection; 2) records indicating subcutaneous or subgaleal abscess; 3) radiologic records related to subcutaneous or subgaleal or epidural or subdural empyema; 4) surgical records indicating bone flap removal or wound revision and irrigation. The event date was determined as the date based on the first reported records indicating cranioplasty infection. If a patient received bilateral cranioplasty over two operations with the second operation occurring at least 2 weeks after the first cranioplasty, the end point of follow-up for the patient was considered to be the date of the second operation. Secondary outcomes measured included bone resorption, epidural hematoma, subdural hematoma, brain contusion, cerebrospinal fluid (CSF) leakage and wound necrosis. Bone resorption was confirmed in cases with a lesion diameter of more than 1 cm, where the remnant thickness of the resorbed cryopreserved graft was less than 50% of that of same region of the contralateral skull. Bone flap resorption was gauged solely on the computed tomography (CT) image. The date of last follow-up brain CT was identified and resorption time was calculated in patients repaired with cryopreserved bone graft. Within 2 weeks after cranioplasty, epidural hematoma or subdural hematoma with a depth of over 0.5 cm on postoperative CT scan, and brain contusion with a diameter greater than 1 cm were evaluated. CSF leakage and wound necrosis within 2 weeks after cranioplasty were also recorded. Mechanical complications included epidural hematoma and subdural hematoma. Regardless of the interval of hospital visits, the patient was considered as having no event unless there were specific records indicating cranioplasty infection.
We undertook analyses of the event-free survival rate of cranioplasty infection according to the type of graft material and the time interval between craniectomy and cranioplasty using the Kaplan-Meier method. The age at craniectomy or cranioplasty was dichotomized as below or above 60 years old. Event-free survival rate of graft infection for cryopreserved bone according to sterilization methods was also performed using the Kaplan-Meier curve. Neurologic outcome at cranioplasty was classified as either good (GOS 4 or 5) or poor (GOS 2 or 3). Potential factors associated with cranioplasty infection were extracted with a univariate Cox regression analysis. The suggested factors were then entered into Cox regression models to estimate the hazard ratio (HR) and 95% confidence intervals for the dichotomous outcome of cranioplasty infection. A p-value of less than 0.05 was considered as statistically significant for all variables. All statistical analyses were conducted using PASW statistics 18.0 (IBM Cor., Armonk, NY, USA).
The selection criteria for the study participants are shown in Fig. 1. The baseline demographic characteristics of the 131 patients undergoing 134 cranioplasties are displayed in Table 1. There was a higher proportion of men compared to women (62.5% vs. 37.5%) and many patients had a history of medical problems (41.2%). The mean ages at craniectomy and cranioplasty were 49.7 and 50.1 years, respectively. The causes of decompressive craniectomy, in order of frequency, included vascular lesions (47.3%), head trauma (46.5%) and brain tumor (6.1%). The location of craniectomy was either right (56.5%), left (41.2%) or bilateral (2.3%). A total of 83 patients received autogenous graft materials (63.3%), which were all cryopreserved bone grafts. Artificial bone materials were used in 48 patients (36.6%); of these, 27 patients were repaired with polymethylmethacrylate (PMMA), 12 patients reconstructed with porous polyethylene (Medpor; Porex Surgical, Newnan, GA, USA) and 9 patients with bone cement. The mean duration of cranioplasty surgery was 2.9 hours. Neurologic outcomes at cranioplasty indicated good recovery in 76 patients (58%), moderate disability in 24 (18.3%), severe disability in 15 (11.5%) and a vegetative state in 16 (12.2%). The duration of prescription for prophylactic antibiotics was more than 1 week in 113 patients (86.3%) and less than 1 week in 18 (13.7%). The order of type of administered prophylactic antibiotics was third generation, first generation and second generation cephalosporin. The time interval between craniectomy and cranioplasty was less than 3 months in 84 patients (64.1%) and over 3 months in 47 (35.9%).
Mean event-free survival durations for patients receiving cryopreserved and artificial bone grafts for cranioplasty infection were 3211 and 3539 days, respectively. There was no significant difference in long-term outcome for cranioplasty infection between cryopreserved and artificial bone grafts in the Kaplan-Meier event-free survival curve (p=0.074) (Fig. 2A). The mean event-free survival times based on early or late repair for site infection were 3200 and 3529 days, respectively; there were no significant differences between the two groups (p=0.083) (Fig. 2B). In an analysis of cranioplasty infection rate in the subgroup of the cryopreserved graft group that received sterilization with or without EO gas, no statistical significance was found (p=0.146) (Fig. 2C). Cox-regression analysis showed that only the patient's neurologic outcome at the time of cranioplasty was significantly related to cranioplasty infection (p=0.04) (Table 2). The patients with poor outcome versus good outcome at cranioplasty showed a HR of 5.203 with a 95% CI of 1.075 to 25.193 for cranioplasty infection (Table 2). The HR for site infection between late cranioplasty versus early cranioplasty was 0.502 (95% CI, 0.096-2.624, p=0.414). The HR for cranioplasty infection of an artificial graft versus cryopreserved bone was 0.303 (95% CI, 0.061-1.516, p=0.146). Although other variables, including age, sex, cause of craniectomy, medical problems, and mechanical complications failed to show statistical significance in this model, we observed a trend between a shorter duration of cranioplasty and site infection (p=0.088) (Table 2). The profiles of 14 patients with cranioplasty infection are summarized in Table 3. The overall incidence of cranioplasty infection was 10.6% and the mean duration to infection was 71.5 days (range, 4-629 days). The incidence rates for site infection in cryopreserved and artificial bone grafts were 14.4% and 4.2%, respectively. The incidence rates for site infection for early and late repair were also 14.4% and 4.2%. Within 2 weeks, 8 of 14 patients (57.1%) showed infection after cranial repair. Cultured microorganisms at the infection site were methicillin-resistant Staphylococcus aureus in 6 patients, methicillin-resistant coagulase-negative staphylococci in 3, Staphylococcus aureus in 1, Pseudomonas aeruginosa in 1, Enterobacter aerogenes in 1, Staphylococcus chromogenes in 1, and Candida guilliermondii in 1. Acute cranioplasty infection within 2 weeks occurred in 8 patients and chronic infection over 2 weeks occurred in 6. Seven of the patients with cranioplasty infection were treated via removal of the infected bone graft. One patient underwent wound revision and abscess removal. The other 7 patients with site infections were successfully treated with antibiotics only (Table 3).
Postoperative complications within 2 weeks after cranioplasty are shown in Fig. 3. Epidural hematoma, subdural hematoma and brain contusion occurred postoperatively within 2 weeks, but none of these events required surgical intervention. There were 77 patients who underwent postoperative brain CT among 83 patients repaired with cryopreserved bone graft. Mean follow-up duration of brain CT scanning of 77 patients was 480 days (range, 1-3361). Resorption of the cryopreserved bone graft occurred in 15 patients (19.4%). The median time from cranioplasty to bone resorption was 834 days (range, 207-3152). Sterilization methods did not affect the resorption rate of the cryopreserved bone graft (Table 4).
The present study showed no differences in the event-free survival rate for cranioplasty infection between cryopreserved and artificial bone material, or between early and late cranioplasty (Fig. 2). However, artificial bone material and late cranioplasty showed a tendency toward better outcomes for cranioplasty infection than cryopreserved bone graft and early cranioplasty, respectively (Fig. 2). After adjusting the potential variables through Cox regression analysis, only neurologic outcome at cranioplasty was significantly related to cranioplasty infection (Table 2). All cranioplasty infection occurred in patients who underwent cranioplasty within 4 months after craniectomy (Table 3).
Yadla et al.23) recently reported in a systematic review that early surgery, implant material, and method of flap preservation had no effect on the rate of cranioplasty infections. However, their review was conducted with nonprospective or nonrandomized study with low-quality23). Chang et al.3) reported a lower incidence of infection rate in autogenous bone grafts (4.6%) compared to artificial bone grafts (18.4%) (p=0.002); however, the definition of cranioplasty infection and the preservation methods for the autogenous bone material were not described. Cheng et al.4) also reported the infection rates for cryopreserved bone graft and PMMA cranioplasty as 13.5% and 6.25%, respectively, without significant difference; however, the definition of graft infection was defined as cases requiring removal of infected grafts. Matsuno et al.15) found a higher site infection rate of 25.9% for autogenous bone and 12.7% for PMMA than 2.6% for custom-made titanium mesh. In our results, cryopreserved autogenous bone grafts had a tendency to more frequently result in the surgical site infection than artificial bone grafts (Fig. 2A). However, the Cox regression model indicated that graft material did not significantly affect cranioplasty infection (Table 2). In the artificial bone graft group, no infection was observed in patients repaired with porous polyethylene (Table 3). It remains unclear whether graft material influences cranioplasty infection after decompressive craniectomy.
Regarding cranioplasty timing after craniectomy, the difference in the infection rate between early (≤3 months) and late (>3 months) cranioplasty tended toward significance (Fig. 2B) likely as other studies4,17). A short time interval was suggested as a risk factor for surgical site infection after cranioplasty in some studies4,17,22), whereas very early cranioplasty within 1 month was preferred in another study13). According to cut-off point of cranioplasty timing, the infection rate between early or late repair could be different including our results13,22). Thavarajah et al.22) reported that cranioplasty conducted at least 6 months post-craniectomy limited the risk of infection. Cranioplasty timing in all patients with surgical site infection occurred within 4 months after craniectomy (Table 3). After Cox regression, statistical significance about cranioplasty timing was not shown and this may be caused due to the small sample size and the cut-off point of early and late cranioplasty. In a recent systematic review, if the cut-off point were more delayed time, the result might be changed23). These finding support the idea that there may be a necessary latency period after decompressive craniectomy to ensure a sufficient wound healing process before cranioplasty should be conducted. Therefore, in spite of statistical insignificance, surgical timing of cranioplasty had better be delayed about 4 or 6 months later after craniectomy.
The factors associated with cranioplasty infection included previous multiple operations, preservation method of autogenous bone material, cause of craniectomy, operative duration of cranioplasty, dysfunction of subgaleal drainage and neurologic status4,9). Risk factors for general postoperative neurosurgical infections include altered sensoria, multiple operations, preexisting infection, emergency surgery, duration of surgery greater than 4 hours, urinary catheterization, CSF leakage, ventilator support, clean-contaminated and dirty surgery, recent neurosurgery, male gender, surgeon, early re-operation, and complexity of surgery and poor neurological outcome13,18,19,22). After adjusting the possible risk factors, poor neurologic condition was the only independent predictor for surgical site infection following cranioplasty identified in the current study (p=0.04). The relationship between a patient's neurologic condition at cranioplasty and infection has not been previously examined, other than in one study that failed to show a significant statistical association between neurologic status and graft infection4). A patient with poor neurologic condition would likely have undergone multiple procedures before cranioplasty or presented with poor nutritional status. In the current study we did not investigate the number of operations before cranioplasty, so our results should be considered cautiously. Mean duration of cranioplasty in the current study was 2.9 hours similar with a previous study (173±10 minutes)7) and shorter than other study9). A shorter duration of cranioplasty might also increase paradoxically cranioplasty infection, as we observed a trend toward significance in the present study (p=0.088). Our result was inconsistent with that of the previous result9). This finding might be biased from measurement of anesthesia time as operation time and sampling error.
The overall infection rates of cranioplasty range from 2.0% to 12.1% in several studies published over the last two decades2-4,9-16). Our infection rate was higher than the mean values observed in other studies, which likely originates from a different definition of cranioplasty infection. If the definition of surgical site infection was confined to removal of bone flap, our infection rate would be 5.4%, which is more similar to those reported in previous studies. The interval between cranioplasty and the onset of infection ranged from 4 to 629 days. Thirteen of 14 cranioplasty infections occurred within 4 months (92.9%), and the remaining infection occurred at 21 months post-surgery. Regardless of material and time interval to cranioplasty, we observed a good long-term event-free survival rate for cranioplasty infection, similar to prior studies22). All microorganisms from pus or wound swabs were gram-positive bacteria in our series except for two gram-negative species and one yeast. Most of the cultured microorganisms were normal skin flora and are comparable to those seen in other studies4,9,10,12,22). Cheng et al.4) showed that the bacterial species from the bone culture before cranioplasty and the wound culture were different. These findings support the idea that cranioplasty infection may originate primarily from wound problem.
The incidence of cryopreserved bone resorption in the present study was similar to that seen in other studies, ranging from 13.6% to 22%1,2,8). Our study did not show a significant difference in bone resorption rates between EO gas sterilization and chemical sterilization in a subgroup analysis. However, we did not perform follow-up brain CT scans routinely, and this likely biases our bone resorption results. Shoakazemi et al.21) observed a low resorption rate of 2%, but their result may originate from the fact that they reported only 2 unacceptable bone flap resorptions after replacement. We did not investigate complaints of scalp indentation after cranioplasty, which is another limitation of the present study. Considering the reporting bias due to selective CT scanning, our true resorption rate may be higher than the reported result. Resorption after cranioplasty with autogenous bone flap is a problem that merits further investigation.
As a retrospective cohort study, the present research contained information and reporting biases due to uncontrolled follow-up schedules and possible underreporting. Additionally, other postoperative issues such as syndrome of the trephined, seizure and hydrocephalus after cranioplasty were not investigated in our study. A cut-off point of cranioplasty timing and neurologic status at cranioplasty may be important issues as identified in the present study. These may be helpful in designing a prospective trial to elucidate long-term prognosis and outcome predictors of cranioplasty complications including surgical site infection following decompressive craniectomy.
Though we did not find sufficient evidence to show that graft material and cranioplasty timing significantly affect the rate of cranioplasty infection following craniectomy, there may be still important factors to be considered before cranioplasty. In patients with poor neurologic status, cranioplasty should be performed carefully to prevent surgical site infection. To more definitively elucidate which factors affect cranioplasty infection, a prospective controlled study is necessary.
References
1. Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006; 104:469–479. PMID: 16619648.

2. Asano Y, Ryuke Y, Hasuo M, Simosawa S. [Cranioplasty using cryopreserved autogenous bone]. No To Shinkei. 1993; 45:1145–1150. PMID: 8123304.
3. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010; 112:1120–1124. PMID: 19612971.

4. Cheng YK, Weng HH, Yang JT, Lee MH, Wang TC, Chang CN. Factors affecting graft infection after cranioplasty. J Clin Neurosci. 2008; 15:1115–1119. PMID: 18656363.

5. Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT. Cranioplasty : cosmetic or therapeutic? Surg Neurol. 1997; 47:238–241. PMID: 9068693.
6. Fiorot JA Jr, Silva GS, Cavalheiro S, Massaro AR. Use of decompressive craniectomy in the treatment of hemispheric infarction. Arq Neuropsiquiatr. 2008; 66:204–208. PMID: 18545783.

7. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy : analysis of 62 cases. Neurosurg Focus. 2009; 26:E9. PMID: 19485722.
8. Honeybul S, Ho KM. Long-term complications of decompressive craniectomy for head injury. J Neurotrauma. 2011; 28:929–935. PMID: 21091342.

9. Huang YH, Yang TM, Lee TC, Chen WF, Yang KY. Acute autologous bone flap infection after cranioplasty for postinjury decompressive craniectomy. Injury. 2011; [Epub ahead of print].

10. Inamasu J, Kuramae T, Nakatsukasa M. Does difference in the storage method of bone flaps after decompressive craniectomy affect the incidence of surgical site infection after cranioplasty? Comparison between subcutaneous pocket and cryopreservation. J Trauma. 2010; 68:183–187. discussion 187. PMID: 20065773.

11. Iwama T, Yamada J, Imai S, Shinoda J, Funakoshi T, Sakai N. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 2003; 52:591–596. discussion 595-596. PMID: 12590683.

12. Jho DH, Neckrysh S, Hardman J, Charbel FT, Amin-Hanjani S. Ethylene oxide gas sterilization : a simple technique for storing explanted skull bone. Technical note. J Neurosurg. 2007; 107:440–445. PMID: 17695404.

13. Kim YW, Yoo DS, Kim DS, Huh PW, Cho KS, Kim JG, et al. The infection rate in case of cranioplasty according to used materials and skull defect duration. J Korean Neurosurg Soc. 2001; 30:S216–S220.
14. Lee SC, Wu CT, Lee ST, Chen PJ. Cranioplasty using polymethyl methacrylate prostheses. J Clin Neurosci. 2009; 16:56–63. PMID: 19046734.

15. Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M, et al. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir (Wien). 2006; 148:535–540. discussion 540. PMID: 16467959.

16. Murthy JM, Chowdary GV, Murthy TV, Bhasha PS, Naryanan TJ. Decompressive craniectomy with clot evacuation in large hemispheric hypertensive intracerebral hemorrhage. Neurocrit Care. 2005; 2:258–262. PMID: 16159072.

17. Nagayama K, Yoshikawa G, Somekawa K, Kohno M, Segawa H, Sano K, et al. [Cranioplasty using the patient's autogenous bone preserved by freezing--an examination of post-operative infection rates]. No Shinkei Geka. 2002; 30:165–169. PMID: 11857940.
18. Otani N, Takasato Y, Masaoka H, Hayakawa T, Yoshino Y, Yatsushige H, et al. Surgical outcome following decompressive craniectomy for poor-grade aneurysmal subarachnoid hemorrhage in patients with associated massive intracerebral or Sylvian hematomas. Cerebrovasc Dis. 2008; 26:612–617. PMID: 18946217.

19. Patir R, Mahapatra AK, Banerji AK. Risk factors in postoperative neurosurgical infection. A prospective study. Acta Neurochir (Wien). 1992; 119:80–84. PMID: 1481758.
20. Qiu W, Guo C, Shen H, Chen K, Wen L, Huang H, et al. Effects of unilateral decompressive craniectomy on patients with unilateral acute post-traumatic brain swelling after severe traumatic brain injury. Crit Care. 2009; 13:R185. PMID: 19930556.

21. Shoakazemi A, Flannery T, McConnell RS. Long-term outcome of subcutaneously preserved autologous cranioplasty. Neurosurgery. 2009; 65:505–510. discussion 510. PMID: 19687696.

22. Thavarajah D, De Lacy P, Hussien A, Sugar A. The minimum time for cranioplasty insertion from craniectomy is six months to reduce risk of infection--a case series of 82 patients. Br J Neurosurg. 2012; 26:78–80. PMID: 21973063.

23. Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD. Effect of early surgery, material, and method of flap preservation on cranioplasty infections : a systematic review. Neurosurgery. 2011; 68:1124–1129. discussion 1130. PMID: 21242830.
24. Zhao Z, Yu J, Liao S, Xiong L, Liang Z, Ling L, et al. Delayed decompressive craniectomy improves the long-term outcomes in hypertensive rats with space-occupying cerebral infarction. Neurocrit Care. 2007; 7:263–269. PMID: 17701109.

Fig. 2
Kaplan-Meier event-free survival curves at 10 years for cranioplasty infection according to graft material (p=0.074) (A), and cranioplasty timing (p=0.083) (B) in 131 cranioplasty patients, and according to sterilization methods (p=0.146) (C) in 83 patients who underwent cranioplasty with a cryopreserved bone graft.
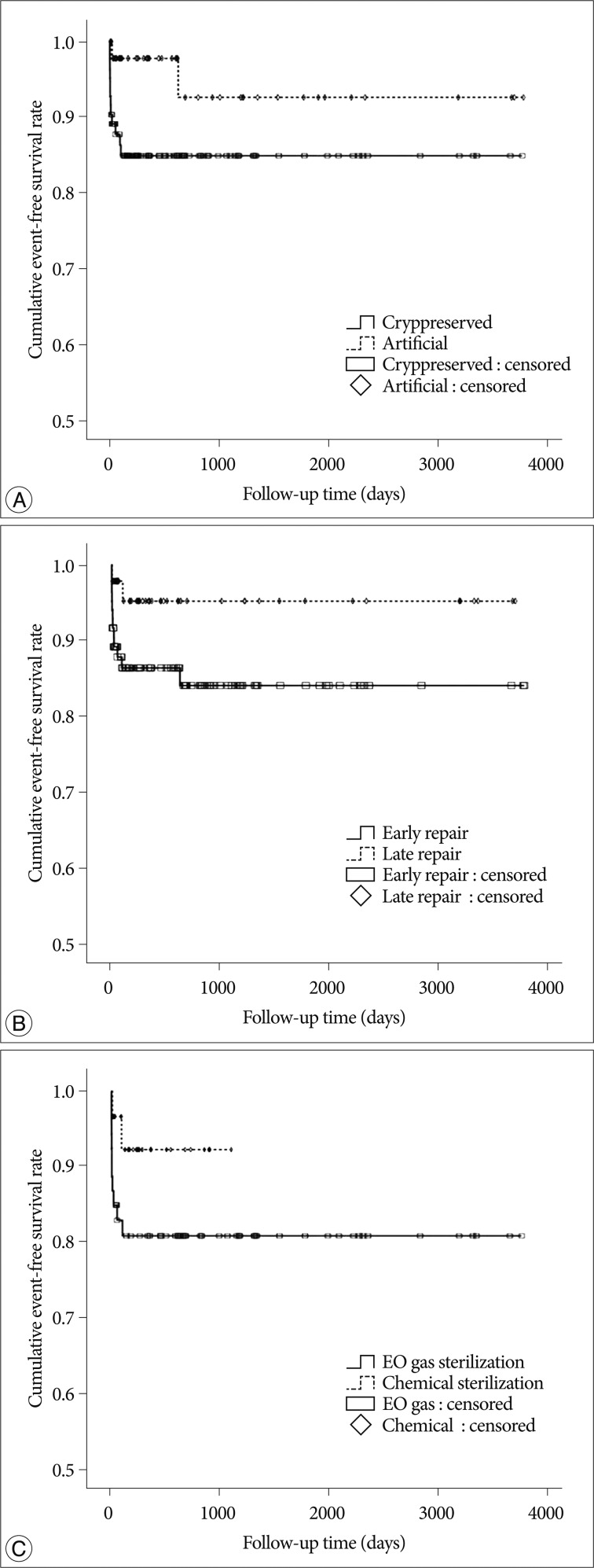
Fig. 3
Postoperative complications within 2 weeks after cranioplasty. EDH, SDH, NA and NC stand for epidural hematoma, subdural hematoma, not applicable and not checked, respectively. Numbers in parentheses indicate the number of patients.
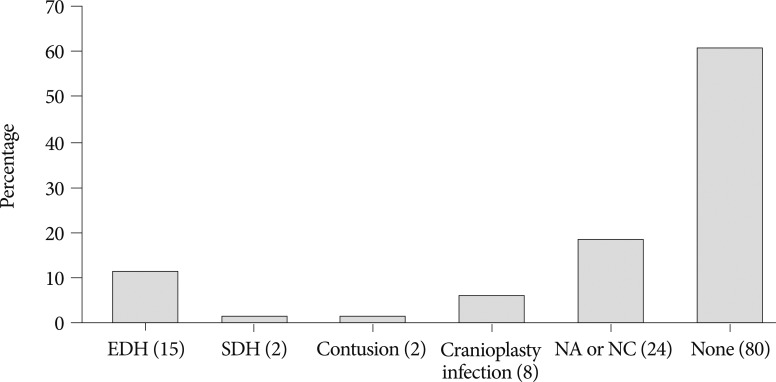
Table 3
Summary of 14 patients with cranioplasty infection after decompressive craniectomy
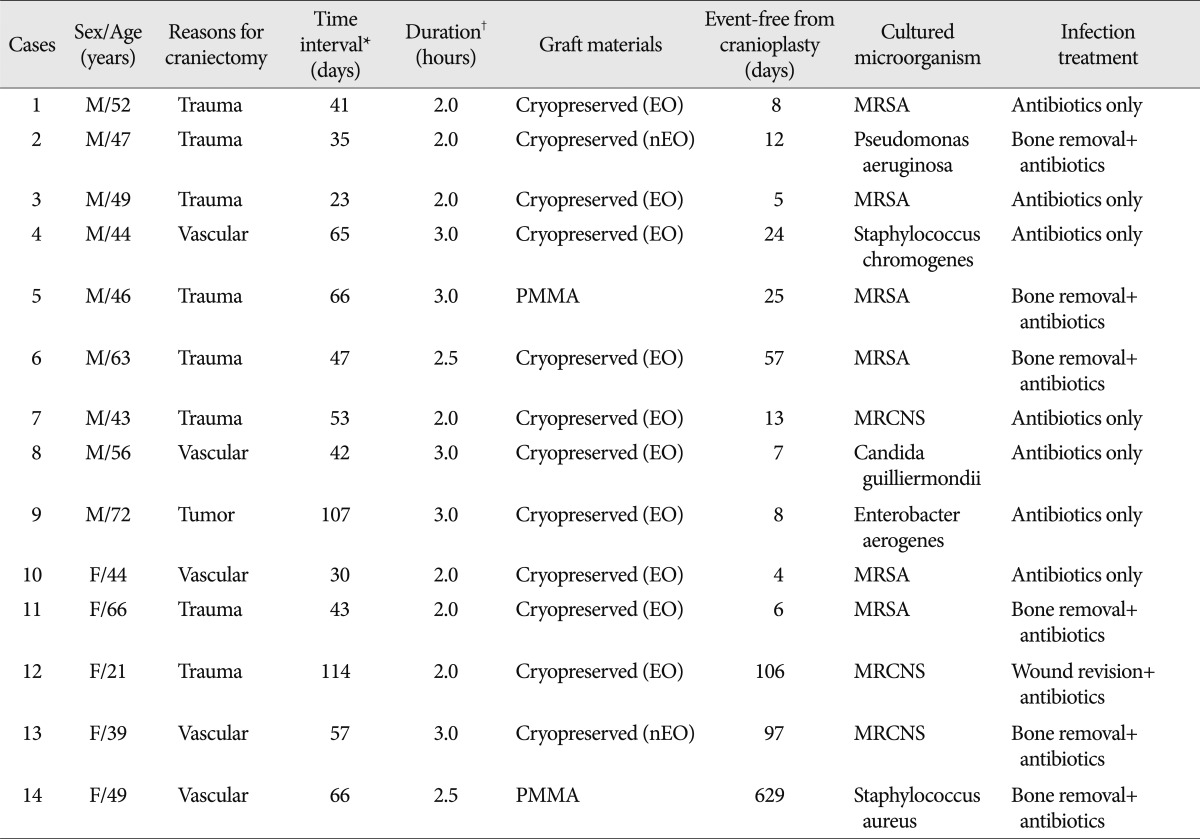
*Time interval indicates the interval between craniectomy and first cranioplasty, †Duration indicates the surgical time to perform a cranioplasty. PMMA : polymethylmethacrylate, EO : sterilization with ethylene oxide before bone fixation, nEO : sterilization without ethylene oxide before bone fixation, MRSA : methicillin-resistant Staphylococcus aureus, MRCNS : methicillin resistant coagulase-negative Staphylococcus




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download