Abstract
The occurrence of symptomatic pituitary hemorrhage into a Rathke's cleft cyst (RCC) is extremely rare. The author reports an interesting case of intra- and suprasellar RCC presented with features of pituitary apoplexy. This 62-year-old woman suffered acute headache, mental confusion, and partial hypopituitarism. The characteristics of the magnetic resonance imaging seemed most compatible with a hemorrhagic pituitary adenoma. Transsphenoidal drainage of the cyst contents confirmed the diagnosis of hemorrhagic RCC and resolved the symptoms. All published data on this rare clinical entity are extracted and reviewed.
Rathke's cleft cysts (RCCs) are benign epithelial lesions of the sella that arise from the remnants of Rathke's pouch. Most RCCs remain small and clinically silent throughout an individual's life. Infrequently, however, the cysts grow and compress the pituitary and surrounding structures, becoming symptomatic. Symptomatic patients having RCCs usually manifest headaches, endocrinopathies, and visual disturbances secondary to suprasellar extension4). Rarely, RCCs can present in a manner similar to pituitary tumor apoplexy10,14,15). One such case is reported. The author also reviews published reports on RCCs presented with apoplexy and describes the clinical, pathological, and radiological characteristics of this rare entity.
A 62-year-old woman developed an acute-onset headache that worsened over 3 days, along with anorexia and dizziness, and she presented to the emergency department for evaluation. The patient was cooperative, but slightly confused. Neurological examination revealed no focal deficit including oculomotor palsy or visual field defect and there was no evidence of neck stiffness. Laboratory results revealed mild hyponatremia. There was no medical history of polyuria, lethargy, and cold intolerance. A complete pituitary endocrine status was assessed, and the patient underwent magnetic resonance (MR) imaging of the sella with and without contrast injection.
Initial MR images demonstrated a 26 mm-sized sellar mass with suprasellar component of heterogeneous signal intensity with peripheral rim enhancement. The lesion had mixed signal intensities on both T1- and T2-weighted scans, with an intracystic fluid level (Fig. 1). These findings suggested the presence of hemorrhage in a pituitary adenoma, RCC, or craniopharyngioma. There were no abnormal vascular lesions on the neuroimages. Pituitary endocrionological findings indicated partial hypopituitarism : the levels of follicle-stimulating hormone, luteinizing hormone, and growth hormone were normal, but thyroid-stimulating hormone, adrenocorticotropic hormone, and cortisol levels were low. The patient's serum prolactin level was mildly elevated to 50 ng/mL suggesting compression of pituitary stalk.
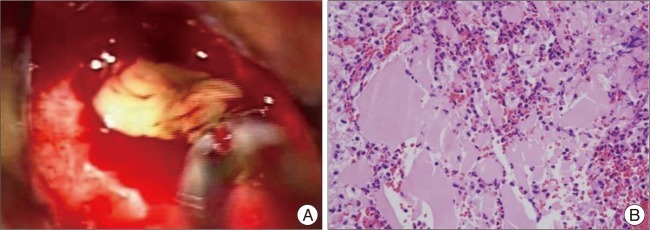
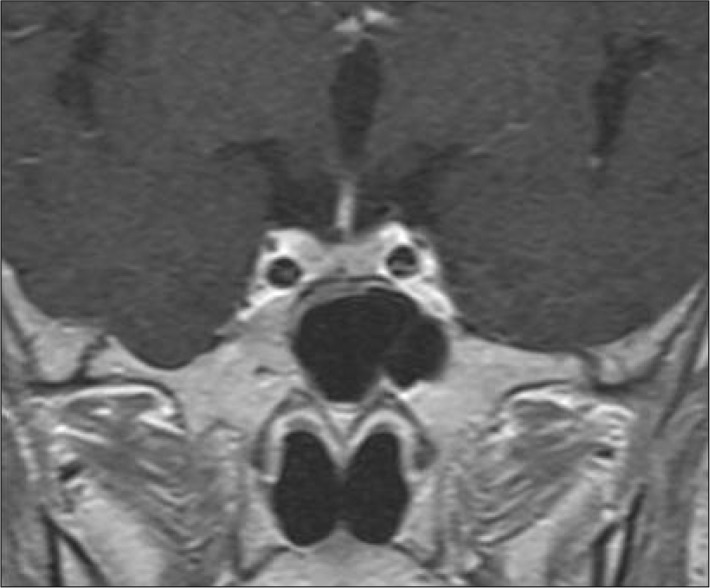
Transsphenoidal microsurgery encountered a bulging of the sella floor which was quite thinned out. Upon opening of the dura mater, there was immediate expression of a bloody serous, mucinous, and yellowish substance (Fig. 2A). Cyst contents were completely evacuated and the cyst wall was partially excised. Histology revealed the lesion to be a sellar cyst with epithelial linings which was suggestive of RCC, as well as evidence of acute hemorrhage mixed in with the contents of the cyst (Fig. 2B). Postoperatively, the patient's headaches disappeared, and the prolactin and sodium levels were normalized. Secondary adrenal deficiency was also resolved 2 months after operation. At 6-year follow-up, MR imaging revealed complete resolution of the lesion (Fig. 3). However, unfortunately, the patient required levothyroxine replacement therapy for hypothyroidism.
Although headaches associated with RCCs are most commonly chronic, some are episodic in nature with frontal focus, possibly indicating an intermittent foreign body reaction caused by mucinous contents12). Acute onset of severe headaches may be associated with hemorrhage within the cyst as well as chemical meningitis, increased sellar pressure, local inflammation8). Occasionally, RCCs cause intracystic abscess, hydrocephalus, hypophysitis, diabetes insipidus, sphenoid sinusitis, oculomotor palsy, hypothalamic dysfunction and even metabolic encephalopathy2,9,12,16).
Only a few reports of pituitary apoplexy associated with RCCs have been described, and their clinical, radiographic, surgical, and histological characteristics are still poorly understood. Recently, the term RCC apoplexy was proposed to define a complex series of clinical events occurring as a consequence of the rapid expansion of the intrasellar contents by hemorrhage within the cyst and the adjacent pituitary tissue7). In fact, however, the confirmatory diagnosis of RCC apoplexy can be made only on pathological confirmation as documented in the present case. Intraoperatively, the surgeons' findings for RCC apoplexy were described macroscopically as coffee-like, xanthochromic, brownish-to-grayish liquid and mucous-yellowish substance with presence of necrotic debris and blood at times12,16). The histological architecture of hemorrhagic RCCs can be often distorted as is also the case in pituitary tumor apoplexy. Usual findings are hemosiderin pigment, cholesterol crystal, inflammatory infiltrate, autolytic change, and blood2,4,15).
The pathogenesis of bleeding into an RCC is not known and unlikely to be explored in a large study in light of the rarity of this condition. Judging from the operative and the histopathologic findings, the authors of recent reports speculated that RCC apoplexy could occur due to hemorrhage from compressed portal veins or newly organized thin-walled vessels within the granulation tissue of the cyst wall especially in large cysts13). There was no apparent known precipitating factor for hemorrhage such as trauma or anticoagulation in any of previously reported cases. It has been suggested that a single layer epithelium of RCCs can make the condition more prone to rupture secondary to minimal arterial pressure variations in some circumstances7).
Although a few authors have analyzed the imaging characteristics of RCCs, it can still be difficult to distinguish the intracystic nodule of an RCC from acute hemorrhage seen in pituitary apoplexy17). This nodule is most commonly appearing as an area within the cyst that exhibit T1-weighted hyperintensity, T2-weighted hypointensity, and no gadolinium enhancement5). In the case series by Binning et al.6), preoperative MR imaging also demonstrated mixed signal intensities suggestive of hemorrhagic pituitary tumor, which intraoperatively was found to be the intracystic nodule of the RCC. In this setting, using a gradient-refocused T2-weighted MR sequence can make the preoperative differentiation between the intracystic nodule and RCC apoplexy less problematic because this modality is helpful in identifying the brain hemorrhage7).
Management of patients who present with a sudden bleeding into a RCC is similar to that of those with pituitary tumor apoplexy. No specific therapy is indicated unless the patient has hypocortisolemia or hypothyroidism, in which case glucocorticoids or thyroid hormones should be administered perioperatively. When bleeding within the RCC is suspected, urgent surgical decompression is warranted, as for pituitary apoplexy caused by pituitary adenoma. Drainage of the cyst contents with clots and a safe excision of the cyst wall through the endonasal transsphenoidal approach is the treatment of choice1). Surgery provides definitive diagnosis and immediate relief of symptoms and often leads to recovery from hypopituitarism as observed in the present case. A review of reported case series suggests that patients with RCC apoplexy require long-term follow-up as clinically indicated to monitor pituitary function and due to the potential for recurrence of an apoplectic episode3,18).
The author reports a rare case of acutely symptomatic RCC which was presented with pituitary apoplexy. The imaging features and treatment strategies of RCC apoplexy are quite similar to those observed in patients with pituitary tumor apoplexy. Patients with hemorrhagic RCC require further research and longer follow-up as clinically indicated to assess pituitary endocrine function and to observe over possible recurrence of an apoplectic episode.
References
1. Aho CJ, Liu C, Zelman V, Couldwell WT, Weiss MH. Surgical outcomes in 118 patients with Rathke cleft cysts. J Neurosurg. 2005; 102:189–193. PMID: 15739543.

2. Albini CH, MacGillivray MH, Fisher JE, Voorhess ML, Klein DM. Triad of hypopituitarism, granulomatous hypophysitis, and ruptured Rathke's cleft cyst. Neurosurgery. 1988; 22(1 Pt 1):133–136. PMID: 3344071.

3. Benveniste RJ, King WA, Walsh J, Lee JS, Naidich TP, Post KD. Surgery for Rathke cleft cysts : technical considerations and outcomes. J Neurosurg. 2004; 101:577–584. PMID: 15481709.
4. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke's cleft cysts : a radiological, surgical and pathological review. Pituitary. 2004; 7:131–137. PMID: 16328563.

5. Binning MJ, Gottfried ON, Osborn AG, Couldwell WT. Rathke cleft cyst intracystic nodule : a characteristic magnetic resonance imaging finding. J Neurosurg. 2005; 103:837–840. PMID: 16304987.

6. Binning MJ, Liu JK, Gannon J, Osborn AG, Couldwell WT. Hemorrhagic and nonhemorrhagic Rathke cleft cysts mimicking pituitary apoplexy. J Neurosurg. 2008; 108:3–8. PMID: 18173304.

7. Chaiban JT, Abdelmannan D, Cohen M, Selman WR, Arafah BM. Rathke cleft cyst apoplexy : a newly characterized distinct clinical entity. J Neurosurg. 2011; 114:318–324. PMID: 20509729.

8. Komatsu F, Tsugu H, Komatsu M, Sakamoto S, Oshiro S, Fukushima T, et al. Clinicopathological characteristics in patients presenting with acute onset of symptoms caused by Rathke's cleft cysts. Acta Neurochir (Wien). 2010; 152:1673–1678. PMID: 20495985.

9. Kurisaka M, Fukui N, Sakamoto T, Mori K, Okada T, Sogabe K. A case of Rathke's cleft cyst with apoplexy. Childs Nerv Syst. 1998; 14:343–347. PMID: 9726587.

10. Lee HJ, Kalnin AJ, Holodny AI, Schulder M, Grigorian A, Sharer LR. Hemorrhagic chondroid chordoma mimicking pituitary apoplexy. Neuroradiology. 1998; 40:720–723. PMID: 9860121.

11. Nishioka H, Haraoka J, Izawa H, Ikeda Y. Headaches associated with Rathke's cleft cyst. Headache. 2006; 46:1580–1586. PMID: 17115992.

12. Nishioka H, Ito H, Miki T, Hashimoto T, Nojima H, Matsumura H. Rathke's cleft cyst with pituitary apoplexy : case report. Neuroradiology. 1999; 41:832–834. PMID: 10602857.

13. Oka H, Kawano N, Suwa T, Yada K, Kan S, Kameya T. Radiological study of symptomatic Rathke's cleft cysts. Neurosurgery. 1994; 35:632–636. discussion 636-637. PMID: 7808605.

14. Onesti ST, Wisniewski T, Post KD. Pituitary hemorrhage into a Rathke's cleft cyst. Neurosurgery. 1990; 27:644–646. PMID: 2234374.

15. Pawar SJ, Sharma RR, Lad SD, Dev E, Devadas RV. Rathke's cleft cyst presenting as pituitary apoplexy. J Clin Neurosci. 2002; 9:76–79. PMID: 11749024.

16. Rosales MY, Smith TW, Safran M. Hemorrhagic Rathke's cleft cyst presenting as diplopia. Endocr Pract. 2004; 10:129–134. PMID: 15256330.

17. Turgut M, Ozsunar Y, Başak S, Güney E, Kir E, Meteoğlu I. Pituitary apoplexy : an overview of 186 cases published during the last century. Acta Neurochir (Wien). 2010; 152:749–761. PMID: 20140630.

18. Wait SD, Garrett MP, Little AS, Killory BD, White WL. Endocrinopathy, vision, headache, and recurrence after transsphenoidal surgery for Rathke cleft cysts. Neurosurgery. 2010; 67:837–843. discussion 843. PMID: 20657318.

Fig. 1
Preoperative magnetic resonance images of a patient presented with pituitary apoplexy. The lesion is mainly isointense with some hyperintense foci on T1-weighted images (A and B). A fluid level (arrow) with isointense signal anteriorly and hypointense signal posteriorly is noted on sagittal T2-weighted image (C). It displays rim enhancement surrounding the cyst following gadolinium infusion (D).
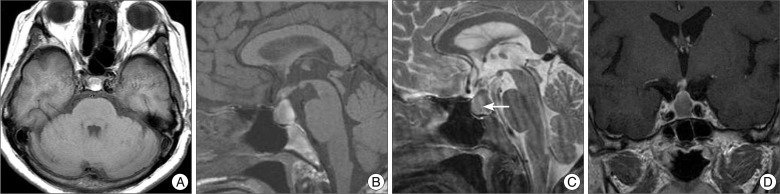
Fig. 2
A : Operative and pathology features of a hemorrhagic Rathke's cleft cyst. At surgery, an initial gush of hemorrhagic fluid was followed by a thick yellowish mucinous substance. B : Histopatholgical examination demonstrates a benign cyst consisting of columnar and cuboidal epitheliums and amorphous cyst contents with acute hemorrhages.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download