Abstract
Objective
The aim of this study was to describe a single center's experience in the management of craniopharyngiomas in children over a 15-year period.
Methods
The clinical records of pediatric patients treated for craniopharyngiomas between December 1995 and February 2011 were reviewed. Thirty-five pediatric patients diagnosed with craniopharyngioma were treated, and their medical records and imaging data were analyzed retrospectively.
Results
The mean follow-up duration was 76 months (range, 10-195). Overall survival and local control rates at 10 years were 94.7±5.1% and 37.1±11.9%, respectively. The female-to-male ratio was 16 : 19, and the mean age was 8.6 years (range, 1-17). Initially, gross total resection (GTR) was performed in 30 patients; subtotal resection (STR) followed by radiotherapy was performed in 5 patients. Of the 14 cases that showed recurrence after GTR, 5 patients were treated with GTR, 1 with radiation therapy (RT), 4 with gamma knife radiosurgery (GKRS), and 4 with subtotal resection followed by RT. No patients who underwent RT or GKRS had recurrences. Two cases with recurrence after STR followed by RT were treated with GTR. One patient died of hormonal insufficiency 64 months after the first surgery. The overall median time progression was 51.2 months (range, 3-182) : 49.7 months in the patients who underwent GTR and 60.2 months in the patients who underwent STR followed by RT.
Craniopharyngiomas are epithelial tumors that originate along the path of the craniopharyngeal duct15). They account for 2% to 5% of all primary intracranial neoplasms and 5.6% to 13% of intracranial tumors in children17). They are classified as intracranial tumors of benign or unspecified behavior by pediatric cancer registries8). Current treatment strategies for craniopharyngiomas include cystic drainage, intratumoral chemotherapy, limited resection, or a combination of gross total resection (GTR) and radiation therapy (RT). Surgery remains the treatment of choice because it allows rapid decompression, minimizes recurrence and provides a histological diagnosis. However, surgery can produce high treatment-related morbidity such as panhypopituitarism, diabetes insipidus, hypothalamic obesity, cognitive deficits4,10), or even death due to the close proximity of crucial neurovascular structures. Regardless of the therapeutic modality chosen, tumor recurrence is common14). Furthermore, secondary surgery to treat recurrent craniopharyngioma is associated with a higher risk of complications and a lower cure rate6). Subtotal resection (STR) combined with RT and radiosurgery (GKRS) is being used increasingly as either a primary or secondary treatment for patients with craniopharyngioma2,12). We report on our experience of treating children with craniopharyngioma at our center.
The clinical records of pediatric patients treated for craniopharyngiomas between December 1995 and February 2011 were reviewed. Thirty-five pediatric patients diagnosed with a craniopharyngioma were treated, and their medical records and imaging data were analyzed retrospectively. Inclusion criteria were as follows : 1) age less than 19 years, and 2) surgical resection with a histopathological diagnosis of craniopharyngioma. The initial tumor diameter was calculated as the average of the longest diameter and the two other diameters vertical to the longest one. The extent of resection was determined by analyzing postoperative computed tomography scans and magnetic resonance imaging scans. A clinical and radiologic follow-up was performed three months after the diagnosis or initial treatment and then at intervals of one to two years thereafter. Progression of craniopharyngioma was defined as tumor growth on sequential imaging with or without associated symptoms. The overall survival and progression-free survival rates were estimated using the Kaplan-Meier method. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, version 18.0, SPSS Inc., Chicago, IL, USA).
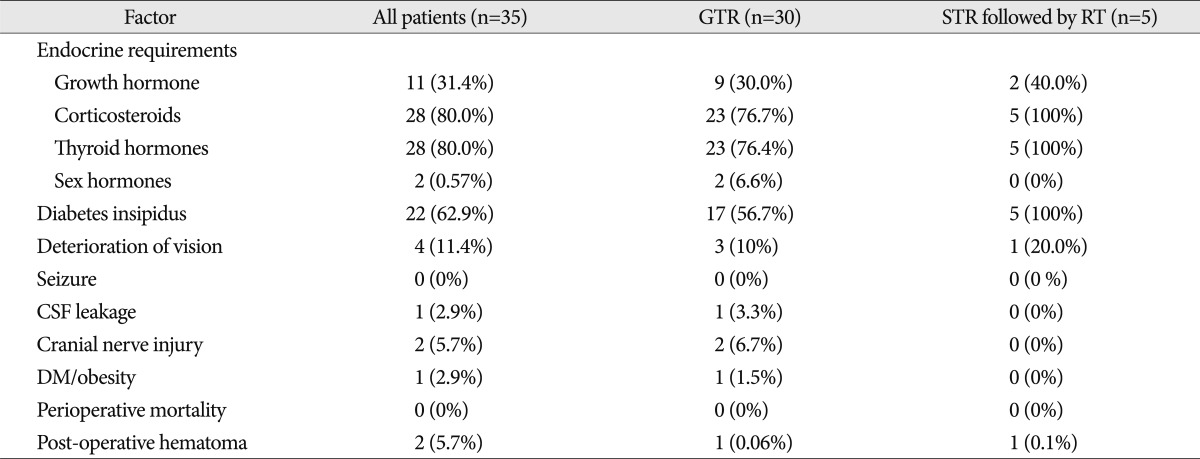
The mean follow-up duration was 76 months (range, 10-195). The female-to-male ratio was 16 : 19, and the mean age was 8.6 years (range, 1-17). The initial mean tumor diameter was 30.4 mm (range, 14-55 mm). Calcification was found in 28 patients. Preoperative tumors were divided into three types : cystic (15 patients), solid (13 patients) and mixed (7 patients). The preoperative tumor location was classified according to Sami's classification16). Eight patients were classified as Grade 1 (intrasellar or infradiaphragmatic) and Grade 2 (occupying the cistern); 8 patients were classified as Grade 3 (lower half of the third ventricle); 13 patients met Grade 4 criteria (upper half of the third ventricle); and 6 patients were Grade 5 (reaching the septum pellucidum or lateral ventricle). The symptoms at initial presentation included visual symptoms (13 patients), such as a visual field defect and decreasing visual acuity, nausea/vomiting (8 patients), endocrine abnormalities (7 patients), headaches (3 patients; 42.9%), dizziness (3 patients), and seizure (1 patient). Seven patients had endocrine abnormalities at the time of initial diagnosis. Baseline characteristics of the 35 patients stratified by initial treatment are summarized in Table 1.
Initially, GTR was performed in 30 patients and STR followed by RT was administered in 5 patients. The RT dosage of patients who underwent STR was 5400 cGy with a median fraction size of 180 cGy.
Twenty-eight patients had endocrine abnormalities that required endocrine hormonal replacement post-treatment. Corticosteroids and thyroid hormone replacement were required in 80.0% of all patients. The frequency of diabetes insipidus in patients undergoing GTR was 56.7% compared with 100% in patients who underwent STR combined with postoperative RT. The difference in endocrine requirements and complications among the groups was not statistically significant because of the small number of cases (Table 2). One patient died of hormonal insufficiency 41 months after the first surgery. Another patient died of hormonal insufficiency 64 months after the first surgery. However, patients did not exhibit severe functional disability.
Progression-free was 51.2 months (range, 3-182): 49.7 months in the patients who underwent GTR and 60.2 months in those who received STR followed by RT (Table 3). Overall survival and local control rates at 10 years were 94.7±5.1% and 37.1±11.9%, respectively (Fig. 1).
In 14 patients (46.7%), the tumor recurred after GTR. The mean time from the initial diagnosis until recurrence after surgery was 32.4 months (range, 5-108). Differences in tumor type and calcification were not significant in patients who had a recurrence. Of 14 cases with a recurrence after GTR, 5 patients were treated with second surgery (GTR), 1 patient with RT, 4 patients with GKRS, and 4 with STR followed by RT.
Recurrence developed in four patients among them who underwent GTR as a secondary treatment. These four patients were treated with GKRS. Remaining one patient died after second surgery because of hormonal insufficiency. The one patient who received RT only was prescribed a dosage of 5000 cGy. No recurrence had developed during the time from RT until the last follow-up. The remaining four patients underwent STR followed by RT; their mean dosage was 3810 cGy (range, 1040-5400 cGy). None of these four patients developed recurrence.
GKRS was administered to four patients. Leksell Gamma Knife types B or C (Elekta Instruments, Atlanta, GA, USA) were used for radiosurgery. The mean marginal dosage was 11.2 Gy (range, 9-14 Gy), and the mean target volume was 1719 mm3 (range, 424-6874). The local control rate of the lesions treated with GKRS was 100% (decreased in two patients and stable in two patients) at the final follow-up (mean, 75 months). A second recurrence did not develop in the patients who underwent RT and GKRS. The local tumor control rates for patients who received RT or GKRS was 100% at relapse (p=0.00) (Fig. 2). Two cases with recurrence after STR followed by RT as an initial treatment were treated with GTR via a transnasal approach. No recurrence had developed during the time from repeat GTR until the last follow-up. The characteristics of recurrent patients are summarized in Table 4.
Current treatment strategies for craniopharyngiomas include cystic drainage, intratumral chemotherapy, limited resection or GTR, and RT. Radical resection at presentation offers the best chance of disease control and potential cure with acceptable morbidity3) because craniopharyngiomas are histologically benign. However, surgery rarely obliterates the tumor completely. The ability to achieve GTR varies between 43-76% in published pediatric series4,10). Furthermore, local recurrence rates have been reported to range from 20-30% after GTR6,9) and from 70-100% after STR without adjuvant treatment10,20,21). To prevent recurrence, aggressive resection is required, which may result in severe permanent neurologic injury and related complications, such as post-treatment endocrinopathy5,7), hypothalamic and frontal lobe damage and hypothalamic obesity4,10). In our series, GTR was achieved in 30 patients (85.7%). The local recurrence rate (14 patients, 46.7%) was slightly high compared with previous reports. The cause of our high local recurrence rate may be a minimal capsule or calcification around the tumor, which was closely adjacent to critical structures.
The fine balance between decreasing neurological deficits and controlling local tumors has led to the use of radiation. Radiation therapies, including fractionated radiation and stereotactic radiosurgery, are often applied postoperatively in the event of STR or tumor recurrence2,7,11,12,18,19).
Fractionated RT improves craniopharyngioma control and survival18) and is the standard treatment for residual or recurrent tumors. Most patient series have demonstrated that when combined with STR, adjuvant radiotherapy allows for greater tumor control and survival than surgery alone19). Dosages of 5000-6000 cGy are most commonly used19). In our study, five patients underwent fractionated RT combined with STR as an initial treatment. The mean dosage was 5400 cGy with a median fraction size of 180 cGy, and the mean tumor size was 37.7 mm (range, 25-45 mm). Recurrence developed in two of these five patients. The mean time to recurrence (16.0 months; range, 3-29) was shorter compared with that of GTR.
Of 14 cases with a recurrence after first surgery (GTR), 9 patients were treated with second surgery. However GTR could be achieved only in 5 patients due to adhesion to surrounding critical structures.
Five patients who experienced recurrence of their tumor after GTR as an initial treatment underwent RT as a secondary treatment. One of these patients underwent RT alone with a dosage of 5000 cGy. No recurrence developed from the time of RT treatment through the last follow-up. The other four patients experiencing a recurrence underwent STR followed by RT. Their mean dosage was 3810 cGy (range, 1040-5400). Recurrence was not noted in these four patients.
Stereotactic radiosurgery is a relatively recent therapeutic option for craniopharyngioma that has significantly improved the effectiveness of and morbidity associated with RT. Kobayashi11) published the largest treatment and outcomes series, which involved 98 cases. At a mean marginal dosage of 11.5 Gy and a mean tumor size of 3.5 cm3, the tumor control rate was 79.6% with a complete response in 19.4% and a partial response in 67.4% of the cases. The actual five- and ten-year survival rates were 94.1% and 91%, respectively, with respective PFS rates of 60.8% and 53.8%. Young age was reported to be a predictor of unfavorable outcome after radiosurgery for craniopharyngioma. In our study, four patients received GKRS. Their mean age was 8.75 years (range, 3-11 years). The mean marginal dosage was 11.2 Gy (range, 9-14 Gy), and the mean target volume was 1719 mm3 (range, 424-6874). The local control rate of the lesions treated with GKRS was 100% (decreased in two patients and stable in two patients) at the final follow-up (mean, 75 months), although all patients were young.
Nevertheless, the use of RT in malignant lesions in children has always posed a therapeutic dilemma; the risk of long-term toxicity must be balanced with the risk of recurrence. This issue is particularly challenging in the management of a benign neoplasm, such as craniopharyngioma, for which overall survival rates are 90-95% at five years and for which side effects from therapy or local progression of the disease can have devastating consequences5).
Treatment-related side effects of RT have been well described in the literature and include endocrine, visual and cognitive sequelae as well as vasculopathy and secondary malignancies1,9,13). Side effects may occur acutely, but they are generally considered to be insidious9). In our study, no newly developed side effects or complications associated with RT or GKRS, such as visual disturbances, endocrine disorders or decreased cognitive function, were observed. This may be because all patients underwent surgery as an initial treatment, and pre-radiation neurologic symptoms were therefore already present.
Limitations of our study include the fact that it was a retrospective, non-randomized analysis with a relatively short follow-up duration and a small number of cases. A single surgeon with considerable experience with craniopharyngioma performed all surgical procedures. While a randomized controlled study is not practical at this time, a population-based prospective study may provide more reasonable evidence-based treatment guidelines in the future.
Optimal management of craniopharyngiomas remains highly debatable. Our results suggest that if safe resection is possible, surgery should be the treatment of choice to prevent tumor recurrence. However, if the tumor is located near critical structures or if it recurs, subtotal resection combined with RT or GKRS may be effective both as an initial treatment for patients with a recurrent tumor and as a salvage treatment for recurrent lesions.
References
1. Aquilina K, Merchant TE, Rodriguez-Galindo C, Ellison DW, Sanford RA, Boop FA. Malignant transformation of irradiated craniopharyngioma in children : report of 2 cases. J Neurosurg Pediatr. 2010; 5:155–161. PMID: 20121363.
2. Chung WY, Pan DH, Shiau CY, Guo WY, Wang LW. Gamma knife radiosurgery for craniopharyngiomas. J Neurosurg. 2000; 93(Suppl 3):47–56. PMID: 11143262.

3. Elliott RE, Hsieh K, Hochm T, Belitskaya-Levy I, Wisoff J, Wisoff JH. Efficacy and safety of radical resection of primary and recurrent craniopharyngiomas in 86 children. J Neurosurg Pediatr. 2010; 5:30–48. PMID: 20043735.

4. Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M. Surgical treatment of craniopharyngiomas : experience with 168 patients. J Neurosurg. 1999; 90:237–250. PMID: 9950494.
5. Fisher PG, Jenab J, Gopldthwaite PT, Tihan T, Wharam MD, Foer DR, et al. Outcomes and failure patterns in childhood craniopharyngiomas. Childs Nerv Syst. 1998; 14:558–563. PMID: 9840379.

6. Gupta DK, Ojha BK, Sarkar C, Mahapatra AK, Sharma BS, Mehta VS. Recurrence in pediatric craniopharyngiomas : analysis of clinical and histological features. Childs Nerv Syst. 2006; 22:50–55. PMID: 15895298.

7. Habrand JL, Ganry O, Couanet D, Rouxel V, Levy-Piedbois C, Pierre-Kahn A, et al. The role of radiation therapy in the management of craniopharyngioma : a 25-year experience and review of the literature. Int J Radiat Oncol Biol Phys. 1999; 44:255–263. PMID: 10760417.

8. Haupt R, Magnani C, Pavanello M, Caruso S, Dama E, Garrè ML. Epidemiological aspects of craniopharyngioma. J Pediatr Endocrinol Metab. 2006; 19(Suppl 1):289–293. PMID: 16700303.
9. Kalapurakal JA. Radiation therapy in the management of pediatric craniopharyngiomas--a review. Childs Nerv Syst. 2005; 21:808–816. PMID: 16075214.

10. Kalapurakal JA, Goldman S, Hsieh YC, Tomita T, Marymont MH. Clinical outcome in children with craniopharyngioma treated with primary surgery and radiotherapy deferred until relapse. Med Pediatr Oncol. 2003; 40:214–218. PMID: 12555247.

11. Kobayashi T. Long-term results of gamma knife radiosurgery for 100 consecutive cases of craniopharyngioma and a treatment strategy. Prog Neurol Surg. 2009; 22:63–76. PMID: 18948720.

12. Lin LL, El Naqa I, Leonard JR, Park TS, Hollander AS, Michalski JM, et al. Long-term outcome in children treated for craniopharyngioma with and without radiotherapy. J Neurosurg Pediatr. 2008; 1:126–130. PMID: 18352781.

13. Merchant TE, Kiehna EN, Sanford RA, Mulhern RK, Thompson SJ, Wilson MW, et al. Craniopharyngioma : the St. Jude Children's Research Hospital experience 1984-2001. Int J Radiat Oncol Biol Phys. 2002; 53:533–542. PMID: 12062594.

14. Ohmori K, Collins J, Fukushima T. Craniopharyngiomas in children. Pediatr Neurosurg. 2007; 43:265–278. PMID: 17627142.

15. Parisi JE, Mena H. Nelson JS, Parisi JE, Schochet SS, editors. Nonglial tumours. Principles and Practice of Neuropathology. 1993. St. Louis: Mosby;p. 203–266.
16. Sammi M, Samii A. Kaye AH, Laws ER, editors. Craniopharyngioma. Operative Neurosurgical Technique. 1995. Philadelphia: WB Sunders;p. 357–370.
17. Schoenberg BS, Schoenberg DG, Christine BW, Gomez MR. The epidemiology of primary intracranial neoplasms of childhood. A population study. Mayo Clin Proc. 1976; 51:51–56. PMID: 1249998.
18. Schubert T, Trippel M, Tacke U, van Velthoven V, Gumpp V, Bartelt S, et al. Neurosurgical treatment strategies in childhood craniopharyngiomas : is less more? Childs Nerv Syst. 2009; 25:1419–1427. PMID: 19714341.

19. Varlotto JM, Flickinger JC, Kondziolka D, Lunsford LD, Deutsch M. External beam irradiation of craniopharyngiomas: long-term analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys. 2002; 54:492–499. PMID: 12243827.

20. Weiss M, Sutton L, Marcial V, Fowble B, Packer R, Zimmerman R, et al. The role of radiation therapy in the management of childhood craniopharyngioma. Int J Radiat Oncol Biol Phys. 1989; 17:1313–1321. PMID: 2689398.

21. Wen BC, Hussey DH, Staples J, Hitchon PW, Jani SK, Vigliotti AP, et al. A comparison of the roles of surgery and radiation therapy in the management of craniopharyngiomas. Int J Radiat Oncol Biol Phys. 1989; 16:17–24. PMID: 2912938.

Fig. 1
Graph showing overall survival and local control rates in 35 patients with craniopharyngiomas over the follow-up period. Overall survival and local control rates at 10 years are 94.7±5.1% and 37.1±11.9%, respectively.

Fig. 2
Graph showing local tumor control rates in 14 patients with recurrence after initial treatment (GTR) stratified by radiation therapy (RT) or radiosurgery received (GKRS) (p=0.00). Five patients are treated with repeat surgery (GTR), 9 patients with RT or GKRS. GTR : gross total resection, GKRS : gamma knife radiosurgery, RT : radiation therapy, STR : subtotal resection.
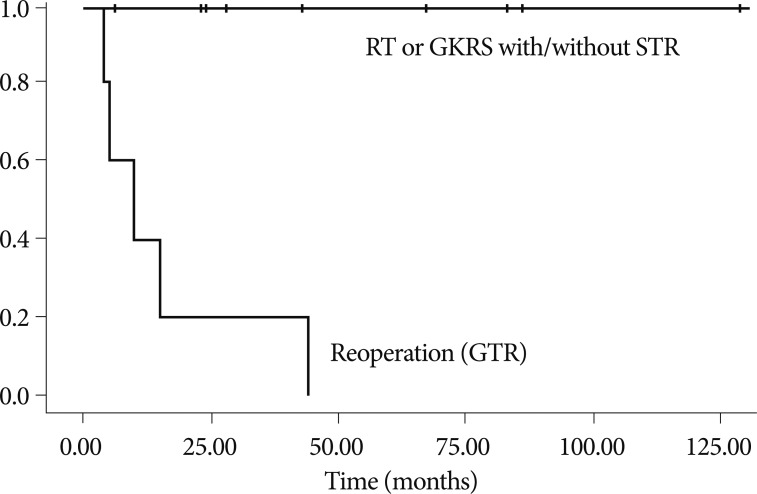
Table 1
Baseline characteristics of 35 children with craniopharyngiomas stratified by initial treatment

Table 2
Complications and endocrine requirements after treatment of 35 patients with craniopharyngioma according to initial treatment methods




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download