Abstract
Objective
The purpose of this large prospective study is to assess the association between the disappearance of the lateral spread response (LSR) before and after microvascular decompression (MVD) and clinical long term results over two years following hemifacial spasm (HFS) treatment.
Methods
Continuous intra-operative monitoring during MVD was performed in 244 consecutive patients with HFS. Patients with persistent LSR after decompression (n=22, 9.0%), without LSR from the start of the surgery (n=4, 1.7%), and with re-operation (n=15, 6.1%) and follow-up loss (n=4, 1.7%) were excluded. For the statistical analysis, patients were categorized into two groups according to the disappearance of their LSR before or after MVD.
Results
Intra-operatively, the LSR was checked during facial electromyogram monitoring in 199 (81.5%) of the 244 patients. The mean follow-up duration was 40.9±6.9 months (range 25-51 months) in all the patients. Among them, the LSR disappeared after the decompression (Group A) in 128 (64.3%) patients; but in the remaining 71 (35.6%) patients, the LSR disappeared before the decompression (Group B). In the post-operative follow-up visits over more than one year, there were significant differences between the clinical outcomes of the two groups (p<0.05).
Hemifacial spasm (HFS), a syndrome that involves unilateral involuntary twitching and contractions of facial muscles, is caused by vascular compression of the facial nerve at the root exit zone. Microvascular decompression (MVD) has been established as an effective surgical procedure for hemifacial spasm1,18). In patients with HFS, the lateral spread response (LSR), produced by electrical stimulation of one branch of the facial nerves, is recorded from facial muscles after facial nerve stimulation. M©ªller and Jannetta12-14) eloquently described the LSR together with the use of an intra-operative evoked electromyogram (EMG) during MVD procedures. Some authors reported that the disappearance or decreased amplitude of the LSR after MVD was associated with postoperative spasm relief or a favorable clinical outcome6,19). On the other hand, other authors suggested that the disappearance or persistence of an abnormal response in the intra-operative monitoring after decompression cannot predict the improvement of facial spasm and its effectiveness may be questionable3,7,9). There have been many reports on the association between LSR findings after decompression and the clinical outcome of hemifacial spasm.
Few reports described the association between the disappearance of LSR before and after MVD and the post-operative result of spasm. Kim et al.8) reported that when comparing two groups based upon whether the LSR disappeared before or after decompression, facial EMG monitoring of the LSR is helpful in predicting outcomes. This factor must be considered a prognostic factor of HFS after MVD, together with the disappearance or persistence of the LSR after decompression. In that paper, the mean follow-up duration was 17.9 months (range : 12-27 months) less. Reports on the correlation between the disappearance or persistence of the LSR after MVD and clinical outcomes usually had a follow-up duration of less than about two years5,7,17). In the authors' institute, their senior colleague (S.H.L.) has also checked continuous intra-operative LSR monitoring during MVD and the clinical outcomes of hemifacial spasm. Furthermore, long-term clinical follow-up data over two years were collected on the intra-operative monitoring results.
It was hypothesized that the disappearance of the LSR before and after MVD also predicts the long-term clinical outcomes over two years. This study was conducted to clarify the long-term effectiveness of intra-operative electromyography during MVD for HFS. In the authors' prospective study, we investigated the association between the disappearance of LSR before and after MVD for the prediction of the short- and long-term clinical outcomes of HFS.
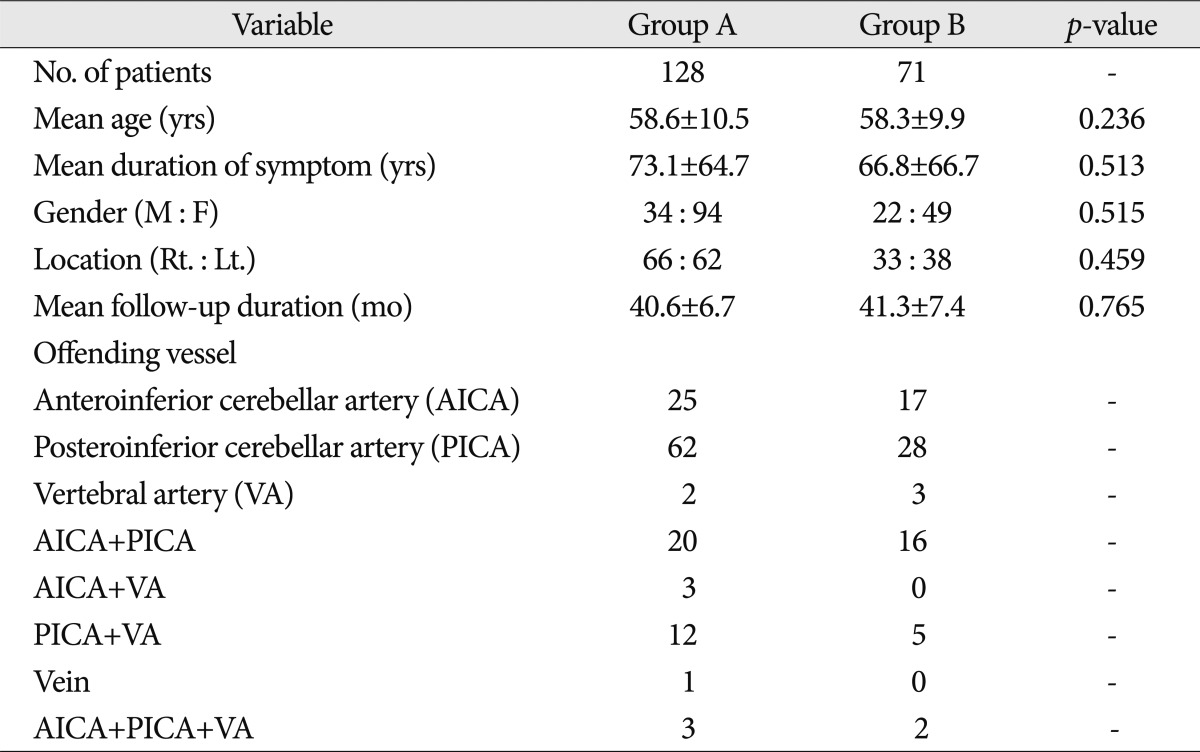
Between June 2006 and December 2008, 244 patients with HFS who underwent MVD were prospectively pooled, together with their intra-operative facial EMG recordings. The patients included those with typical symptoms of HFS, disappearance of an abnormal LSR before or after MVD, and a minimum follow-up period of at least two years were included. Forty-five of the 244 patients were excluded because of persistent LSR after decompression (n=22, 9.0%), the non-existence of an LSR from the start of their surgery (n=4, 1.6%), re-operation (n=15, 6.1%), and follow-up loss (n=4, 1.6%). For the patients in whom no LSR was observed, much effort was made to make the LSR appear, such as the use of a low-dose muscle relaxant or repositioning of the insertion site of the EMG needle. Despite these efforts, the LSR could not be induced in four of the 244 patients (1.6%), although they showed typical HFS. Thus, 199 patients were included in this study. Fifty-six of them were men and 143 were women aged 25-85 years, with a mean value of 58.5±10.3 years. The symptom duration varied from 3 to 480 months, with a mean value of 67.3±65.7 months. The mean follow-up duration was 40.9±6.9 months (range : 25-51 months) (Table 1).
From the time of the administration of general anesthesia to the dural closure, continuous facial EMG was monitored using needle EMG recordings from the orbicularis oculi, orbicularis oris, and mentalis muscles with the Viking IV (Nicolet™). The brainstem auditory evoked potential was also determined in all the patients. Depolarizing muscle relaxants were not used (except before the intubation). Bipolar stainless needle electrodes were subdermally placed in the mentalis muscles. A lateral spread response appeared in the other facial muscles during the subdermal stimulation of the temporal branch of the facial nerve. We used stimuli of a 0.1-0.2 msec pulse wave with an intensity of 5-30 mA. There were nine check points to detect when the LSR disappeared : 1) after the administration of the anesthesia, 2) before the dural opening, 3) immediately after the dural opening, 4) at the time of the CSF drainage, 5) before the decompression, 6) during the dissection, 7) after the decompression, 8) before the dural closure, and 9) after the dural closure. The patients were classified into two groups according to the timing of their LSR disappearance : Group A [in which the LSR disappeared after the decompression (7-9)] and Group B [in which the LSR disappeared before the decompression (1-6)]. Of the 199 patients in whom the LSR disappeared during the surgery, 128 (64.3%) were placed in Group A and 71 (35.7%), in Group B.
One surgeon performed all the microvascular decompression surgery procedures in the authors' institute. All patients were put under general anesthesia using a lateral suboccipital retrosigmoid approach and auditory brainstem evoked potentials, which are well described in the literature10,11,16). After the dura mater was opened and the CSF was drained, appropriate brain relaxation was achieved. Gentle elevation of the cerebellum exposed the compressed root exit zone of the facial nerve. Teflon felt implants were used for the decompression. Water-tight dural closure was performed, with several pieces of muscle interposed between the interrupted sutures.
A statistical analysis was performed with commercial software (SPSS V15.0, SPSS Inc., Chicago, IL, USA). The data are presented as means±standard deviations. A chi-square test was used to assess the statistical significance of the independent variables of the two groups, and an independent t-test was used to compare the degrees of the clinical outcomes of the two groups.
All 199 patients were followed for 40.9 months (range : 25-51 months). The clinical data evaluations were performed one week, three months, one year, two years, and three years after the MVD surgery. To assess the effects of the MVD surgery, a more than 90% improvement in the spasm was defined as complete relief; >50%, partial relief; and a <50% decrease in symptoms or unchanged symptoms, as no relief. In this study, 144 (72.4%) of the 199 patients in whom the LSR disappeared during the surgery showed complete relief of HFS over two years. Over the more than three-year follow-up, 98 (64.1%) of the 153 patients showed complete relief of HFS. None of the patients had intracranial hemorrhage or died. The post-operative complications included transient hearing loss in one patient (0.5%), facial palsy in five patients (2.5%), post-operative wound infection in three patients (1.5%), cerebrospinal fluid rhinorrhea in one patient (0.5%), and tongue sensory change in one patient (0.5%).
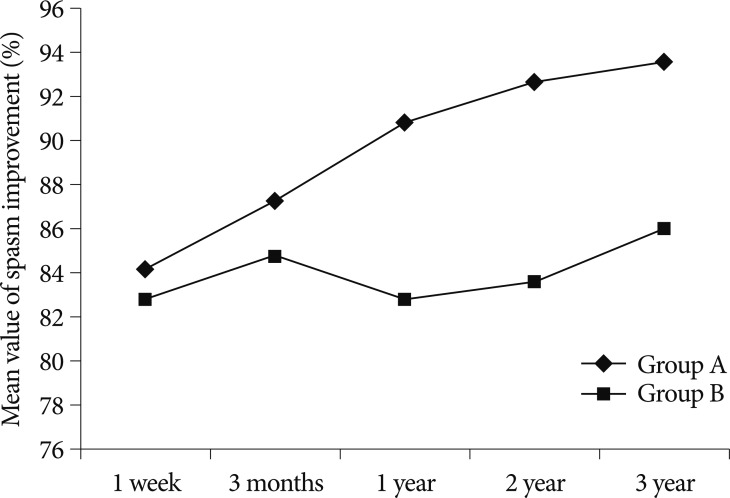
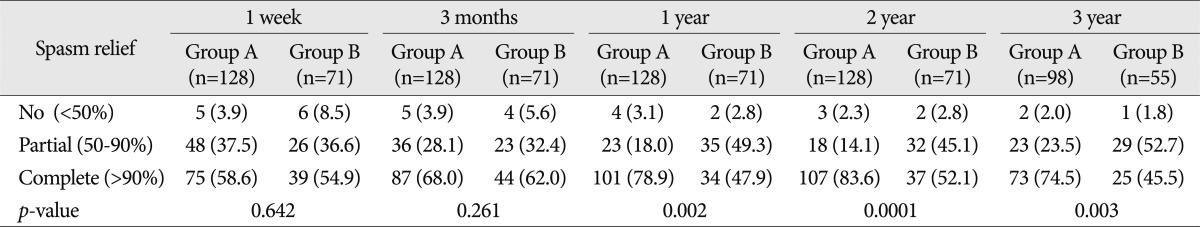
For the analysis of the efficacy of intra-operative facial EMG monitoring, the 199 subject patients were divided into two groups depending on the disappearance of their LSR before or after their decompression. Group A included 128 patients (64.3%) in whom the LSR disappeared after the decompression, and Group B included 71 patients (35.7%) in whom the LSR disappeared before the decompression. In the one-week outcomes after the surgery, HFS was completely relieved in 75 (58.6%) patients in Group A and 39 (54.9%) patients in Group B. At three months post-operatively, complete relief occurred in 87 (68.0%) patients in Group A and 44 (62.0%) patients in Group B. In the one-year follow-up examination, 101 (78.9%) patients in Group A and 34 (47.9%) patients in Group B were completely cured. In the post-operative two-year examination, 107 (83.6%) patients showed complete HFS relief in Group A, and 37 (52.1%) patients showed complete spasm relief in Group B. In the post-operative three-year examination, complete relief occurred in 73 (74.5%) patients in Group A and 25 (45.5%) patients in Group B. The complete relief rate of Group A was higher than that of Group B after one-, two-, and three-year follow-up (Fig. 1). There were statistically significant differences between the one-year (p value=0.002), two-year (p value=0.0001), and three-year (p value=0.003) follow-up results for the two groups. There were no statistically significant differences in the results after one week (p value= 0.642) and three months (p value=0.261) (Table 2).
LSR is usually immediately reduced with decompression of the facial nerve root. There is still much controversy, however, over whether or not intra-operative LSR disappearance means adequate decompression of the facial nerve. Some authors3,7,15) reported that an intra-operative change in the LSR did not always indicate a favorable prognosis. Many authors tried to investigate the correlation between the disappearance or persistence of the LSR after decompression and the clinical outcomes with a mean follow-up duration of about two years5,7,17). To authors' knowledge, two papers report that the disappearance or decreased amplitude of the LSR indicates post-operative spasm resolution with a follow-up duration of over two years6,9).
On the other hand, similar to the authors' hypotheses, some studies have tried to prove spasm-free results by disappearance of the LSR before or after the decompression2,14,15,19). It was reported that LSR disappeared before the decompression of the compressing vessel in two of eight patients in one study2). The outflow of cerebrospinal fluid shifts the neurovascular relation, which is temporarily equivalent to decompression2). Mooij et al.15) deemed the abnormal muscle response (AMR) as indirectly confirming the AMR disappearance in patients after the drainage of the cerebrospinal fluid. Their results showed five (6.8%) patients in which the AMR disappeared before the decompression. These five patients with indirect confirming had a lower rate of cured spasm than 25 (33.8%) patients with guiding in which decompression was followed by disappearance of the AMR. Yamashita et al.19) described the AMR disappearance in 53 of 60 patients after their microsurgery, and in nine patients before the transposition of the offending arteries. Among these nine patients, three (33.3%) showed persistent facial spasm in their immediate results. In the long-term results, however, the nine patients were completely cured. These three reports announced the LSR disappearance before the decompression, but they did not directly compare the LSR disappearance before and after the decompression with the spasm-free outcome, and performed the statistical analysis. Kim et al.8) reported that the 75.6% complete cure rate of Group B (in which the LSR disappeared before the decompression) was much lower than the 92.9% of Group A (in which the LSR disappeared after the decompression). Moreover, the spasm-free outcomes in the three-month and one-year results had statistically significant differences between the two groups (p value <0.05). In the discussion section, it was mentioned that further long-term follow-up evaluation may provide more information regarding the association between intra-operative LSR monitoring and post-operative results.
Fortunately, we had opportunities to analyze the results for the two groups with long-term follow-up periods of more than two years. As mentioned, our study also revealed that the complete relief rate of Group A was higher than that of Group B, not only after a one-year follow-up but also after two- and three-year follow-ups. The post-surgical two- and three-year results also significantly differed between the two groups. In this study, the patients in whom the LSR disappeared before the decompression showed poorer results than those in whom the LSR disappeared after the decompression during the long-term follow-up periods of over two years.
Unlike previous results8), the three-month spasm-free results did not differ significantly. It may take time before the motor nucleus hyper-excitability of the facial nerve decreases and its re-myelination process is completed. Several authors have proposed that in some patients, once the vascular compression is resolved, the motor nucleus hyperactivity starts to decline slowly and normalizes over a few months to a few years4-6).
Considering the lower spasm relief rate in Group B (in which the LSR disappeared before the decompression) than in Group A (in which the LSR disappeared after the decompression), it cannot be intra-operatively confirmed if the LSR disappearance in the patients in Group B was actually due to the decompression of the conflicting vessel, because the LSR disappeared before the decompression. Thus, more careful and adequate decompression between the offending vessel and the facial nerve root exit zone may be needed to improve the clinical outcomes of MVD.
Although the LSR disappearance before MVD had poorer outcomes, it is not based on scientific evidence and cannot be proven logically. Moreover, in these patients, despite the knowledge of the need for more careful and adequate decompression, it is not known how much or where the Teflon felt must be added. Furthermore, a study to determine the scientific cause of this phenomenon may be needed.
In this study, it was found that patients in whom the LSR disappeared after the decompression had better prognoses than those in whom the LSR disappeared before the decompression in the long-term follow-up periods of over two years. Thus, intra-operative facial EMG monitoring is helpful in predicting HFS prognosis and verifying the decompression of the facial nerve.
In patients in whom the LSR disappeared before the decompression, the facial nerve should be more carefully and adequately decompressed.
References
1. Barker FG 2nd, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. J Neurosurg. 1995; 82:201–210. PMID: 7815147.

2. Haines SJ, Torres F. Intraoperative monitoring of the facial nerve during decompressive surgery for hemifacial spasm. J Neurosurg. 1991; 74:254–257. PMID: 1988595.

3. Hatem J, Sindou M, Vial C. Intraoperative monitoring of facial EMG responses during microvascular decompression for hemifacial spasm. Prognostic value for long-term outcome : a study in a 33-patient series. Br J Neurosurg. 2001; 15:496–499. PMID: 11814001.

4. Ishikawa M, Ohira T, Namiki J, Gotoh K, Takase M, Toya S. Electrophysiological investigation of hemifacial spasm : F-waves of the facial muscles. Acta Neurochir (Wien). 1996; 138:24–32. PMID: 8686521.

5. Ishikawa M, Ohira T, Namiki J, Kobayashi M, Takase M, Kawase T, et al. Electrophysiological investigation of hemifacial spasm after microvascular decompression : F waves of the facial muscles, blink reflexes, and abnormal muscle responses. J Neurosurg. 1997; 86:654–661. PMID: 9120630.

6. Isu T, Kamada K, Mabuchi S, Kitaoka A, Ito T, Koiwa M, et al. Intra-operative monitoring by facial electromyographic responses during microvascular decompressive surgery for hemifacial spasm. Acta Neurochir (Wien). 1996; 138:19–23. discussion 23. PMID: 8686520.

7. Joo WI, Lee KJ, Park HK, Chough CK, Rha HK. Prognostic value of intra-operative lateral spread response monitoring during microvascular decompression in patients with hemifacial spasm. J Clin Neurosci. 2008; 15:1335–1339. PMID: 18617405.

8. Kim CH, Kong DS, Lee JA, Kwan-Park . The potential value of the disappearance of the lateral spread response during microvascular decompression for predicting the clinical outcome of hemifacial spasms : a prospective study. Neurosurgery. 2010; 67:1581–1587. discussion 1587-1588. PMID: 21107188.
9. Kiya N, Bannur U, Yamauchi A, Yoshida K, Kato Y, Kanno T. Monitoring of facial evoked EMG for hemifacial spasm : a critical analysis of its prognostic value. Acta Neurochir (Wien). 2001; 143:365–368. PMID: 11437290.

10. Kong DS, Park K, Shin BG, Lee JA, Eum DO. Prognostic value of the lateral spread response for intraoperative electromyography monitoring of the facial musculature during microvascular decompression for hemifacial spasm. J Neurosurg. 2007; 106:384–387. PMID: 17367059.

11. McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves : lessons learned after 4400 operations. J Neurosurg. 1999; 90:1–8. PMID: 10413149.

12. Møller AR, Jannetta PJ. Hemifacial spasm : results of electrophysiologic recording during microvascular decompression operations. Neurology. 1985; 35:969–974. PMID: 4010963.
13. Møller AR, Jannetta PJ. Microvascular decompression in hemifacial spasm : intraoperative electrophysiological observations. Neurosurgery. 1985; 16:612–618. PMID: 4000433.

14. Møller AR, Jannetta PJ. Monitoring facial EMG responses during microvascular decompression operations for hemifacial spasm. J Neurosurg. 1987; 66:681–685. PMID: 3572493.

15. Mooij JJ, Mustafa MK, van Weerden TW. Hemifacial spasm : intraoperative electromyographic monitoring as a guide for microvascular decompression. Neurosurgery. 2001; 49:1365–1370. discussion 1370-1371. PMID: 11846935.
16. Park JS, Kong DS, Lee JA, Park K. Hemifacial spasm : neurovascular compressive patterns and surgical significance. Acta Neurochir (Wien). 2008; 150:235–241. discussion 241. PMID: 18297233.

17. Shin JC, Chung UH, Kim YC, Park CI. Prospective study of microvascular decompression in hemifacial spasm. Neurosurgery. 1997; 40:730–734. discussion 734-735. PMID: 9092846.

18. Sindou MP. Microvascular decompression for primary hemifacial spasm. Importance of intraoperative neurophysiological monitoring. Acta Neurochir (Wien). 2005; 147:1019–1026. discussion 1026. PMID: 16094508.

19. Yamashita S, Kawaguchi T, Fukuda M, Watanabe M, Tanaka R, Kameyama S. Abnormal muscle response monitoring during microvascular decompression for hemifacial spasm. Acta Neurochir (Wien). 2005; 147:933–937. discussion 937-938. PMID: 16010450.

Fig. 1
Mean value of the improved percentage of the spasm compared to the initial symptom according to the follow-up duration. This graph shows remarkable differences in these values between Groups A and B, since the post-operative one-year follow-up.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download