Abstract
Objective
Microvascular decompression (MVD) for hemifacial spasm (HFS) is a safe and effective treatment with favorable outcomes. The purpose of this study was to evaluate the incidence of delayed cranirve ( VI, VII, and VIII ) palsy following MVD and its clinical courses.
Methods
Between January 1998 and December 2009, 1354 patients underwent MVD for HFS at our institution. Of them, 100 patients (7.4%) experienced delayed facial palsy (DFP), one developed sixth nerve palsy, and one patient had delayed hearing loss.
Results
DFP occurred between postoperative day number 2 and 23 (average 11 days). Ninety-two patients (92%) completely recovered; however, House-Brackmann grade II facial weakness remained in eight other patients (8%). The time to recovery averaged 64 days (range, 16 days to 9 months). Delayed isolated sixth nerve palsy recovered spontaneously without any medical or surgical treatment after 8 weeks, while delayed hearing loss did not improve.
Conclusion
Delayed cranial nerve (VI, VII, and VIII) palsies can occur following uncomplicated MVD for HFS. DFP is not an unusual complication after MVD, and prognosis is fairly good. Delayed sixth nerve palsy and delayed hearing loss are extremely rare complications after MVD for HFS. We should consider the possibility of development of these complications during the follow up for MVD.
Hemifacial spasm (HFS) is a paroxysmal movement disorder characterized by involuntary, unilateral, infrequent, hyperactive contractions with vascular compression. It is generally believed that HFS is caused by vascular compression of the facial nerve root exit zone (REZ). Microvascular decompression (MVD) has widely been accepted as the primary treatment modality for HFS. However, immediate postoperative complications of MVD include hearing disturbances, facial palsy, supratentorial subdural hematoma, intracerebellar hematoma with acute hydrocephalus, immediate infarction of the brain stem, and subarachnoid hemorrhage of traumatic aneurismal rupture1,7,10,17,23).
Delayed cranial nerve palsy known as rare complication following MVD. Among them, delayed facial palsy (DFP) reported most commonly. Several reports describe an incidence of DFP following MVD in HFS of 2.8-8.3%10,14,15,20). The purpose of this study was to evaluate the incidence of delayed cranial nerve (VI, VII, and VIII ) palsy following MVD and its clinical courses.
Between January 1998 and December 2009, over 1400 consecutive
patients underwent MVD for HFS at our hospital. Among them, 1354 patients who underwent MVD for HFS with minimum of 6 months follow up were enrolled in this study. The patients consisted of 391 men and 963 women who ranged in age from 19 to 77 years (mean age : 49 years). Symptom duration
ranged from 1 month to 420 years (mean : 68 months). Preoperatively, all patients underwent temporal bone computed tomography, internal auditory canal magnetic resonance imaging,
pure tone audiometry and speech discrimination (PTA-SA). All patients were followed up to evaluate outcomes on postoperative days 1 and 3, at 3 weeks and at 3, 6, and 12 months by the surgeon and an assisting nurse. Our definition of delayed cranial palsy was sudden in onset, usually occurring over a 24-hour period15,20) postoperatively.
All surgeries were performed by a single neurosurgeon (K. Park) at our institution. The retromastoid microsurgical approach was performed while the patient was in the lateral decubitus position as described in the medical literature9,10,12,15,20). After opening the dura mater, gentle retraction of the cerebellum exposed the facial nerve that was compressed. Several (five to ten) Teflon threads (DuPont, Wilmington, DE, USA) were inserted between the facial nerve and the offending vessels8,9,12,16).
During surgery, brainstem auditory evoked potential (BAEP) and facial electromyography (EMG) monitoring were performed from the time of administration of general anesthesia until the time of dural closure. Stimulating needle electrodes were inserted intradermally over the zygomatic branches of the facial nerve, and a 0.1- to 0.2-msec pulse wave with an intensity of 5 to 25 mA was used. Werecorded thelateral spread responses (LSR) during evoked facial EMG monitoring that appeared in the other facial muscles, including the orbicularis oculi, the orbicularis oris, and the mentalis muscles, when we stimulated the nerve that innervated the frontalis muscle. In some cases, overdosage of neuromuscular blockade during anesthesia led to alterations in the LSR phenomenon. Therefore, the anesthesiologist made an effort to achieve minimal use of these agents for maintaining the train-off at a level of at least 0.75. Following MVD, the LSR was found to disappear in most of the patients. When the LSR was found to be persistent despite decompression, we looked for any suspicious underlying causes such as possible compression by another vessel. After confirming that there was no further offending vessel, we added one or two pieces of Teflon felt to the lesion sites9,12).
To identify potential risk factor of DFP, We performed the Mann-Whitney U test for continuous variables and Pearson chi-square test for categorical variables. p<0.05 was considered as statistically significant (version 18.0; SPSS, Inc.; Chicago, IL, USA). We each had only one case of delayed sixth nerve and delayed hearing loss, thus such cases were not included in the statistics.
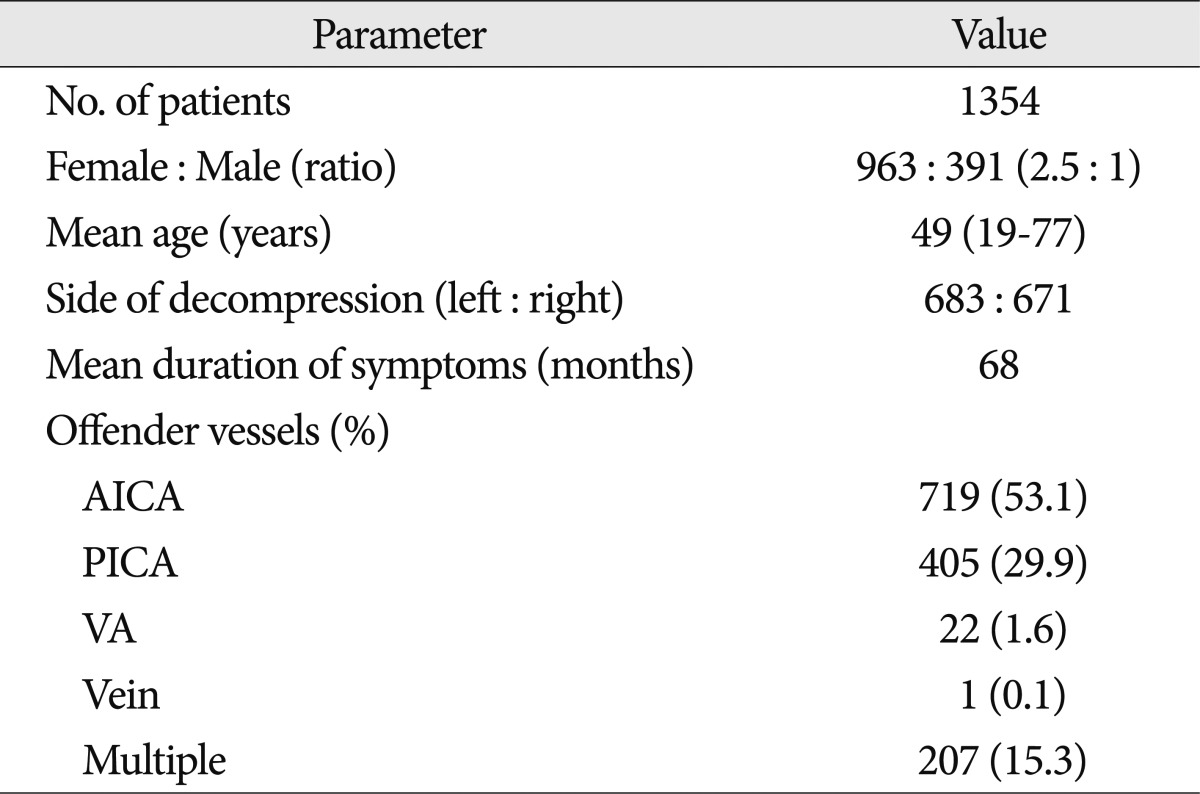
Total 1354 patients performed MVD. A variety of offending vessels were found in all of the 1354 patients. The offending vessels were the anterior-inferior cerebellar artery (AICA) in 670 (53.5%) patients, the posterior-inferior cerebellar artery in 382 (30.4%) patients, the vertebral artery in 21 (1.6%) patients, venous structure in 1 (0.1%) patients, two or more vessels in 180 (14.5%) patients. The right side was affected in 671 patients and the left in 683 patients (Table 1).
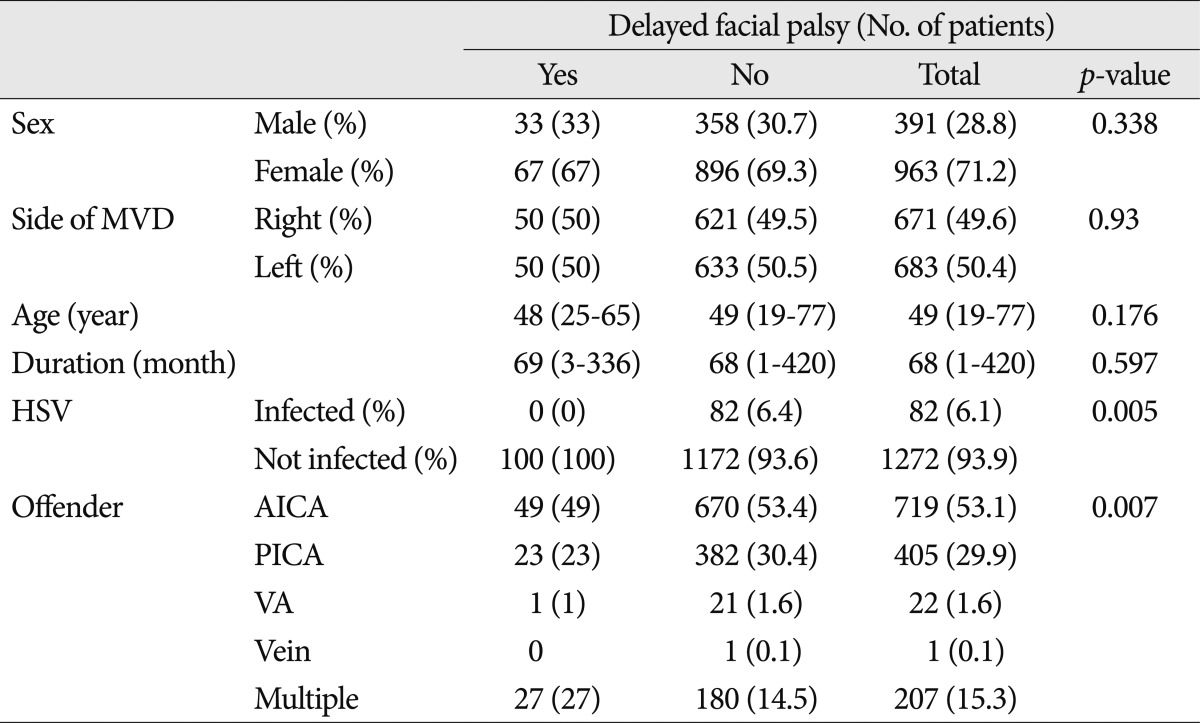
Cranial nerve palsy included immediate facial weakness in 43 (3.2%), immediate hearing loss in 39 (2.89%), delayed facial weakness in 100 (7.4%), one delayed hearing loss (0.1%), one delayed sixth nerve palsy (0.1%). There was no sixth nerve palsy immediately. Among delayed facial palsy patients, there were 33 men and 67 women who ranged in age from 25 to 65 years (mean age : 47.8 years) and the mean duration of symptoms was 5.8 years (range : 3 months-28 years). The onset of palsy occurred in postoperative 11.2 days in average (range : 2-23 days) (Table 2, Fig. 1). The weakness was assessed by the House-Brackmann (HB) scale, and 30 patients were HB Grade II, 54 patients were grade III and patients were grade IV.
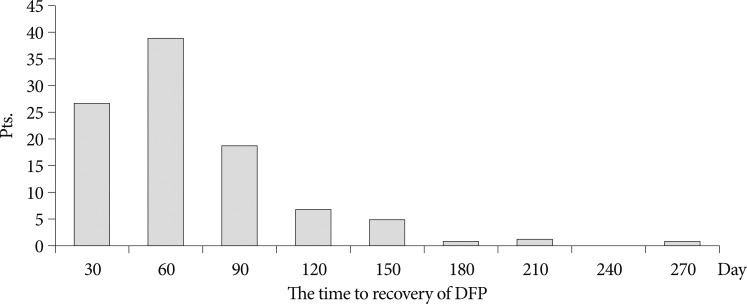
Recently, combined therapy was accepted most generally for DFP, 94 patients were given acyclovir (30 mg/kg/day) and prednisolone (1 mg/kg/day) for at least two weeks21). Eighty-six patients (86%) improved to complete recovery (Grade I) and eight patients improved to minimal weakness (Grade II). The time to recovery averaged 64.1 days (range : 16-270 days) (Fig. 2).
We reviewed one unusual case of delayed hearing loss. A 71-year-old female underwent MVD for HFS. Magnetic resonance imaging showed that the right AICA was in close proximity to the REZ of the ipsilateral facial nerve. The operation was uneventful and BAEP were monitored at a baseline level throughout preparation. Delayed latency and amplitude of Peak V decline occurred during retraction of the cerebellum. Releasing the retractor improved the BAEP, and the BAEP recovered to baseline. After MVD, the patient had no subjective hearing impairment. Three weeks after the MVD, ipsilateral hearing loss occurred.
PTA-SA was performed and showed scale-out on the operated side (Fig. 3). Steroid medication in the form of methylprednisolone 40 mg/mL was given intratympanicly at a dose of 0.5 mL/day over 3 weeks3,22). One month after onset, ipsilateral hearing function improved and the PTA-SA showed a 78 dB deficit on the operated side (Fig. 4). Hearing loss improved a little, but was not cured to the point of achieving serviceable hearing.
We experienced a rare case of isolated sixth nerve palsy. A 50-year-old woman underwent MVD for right-sided HFS. The procedure was uneventful. Three weeks after MVD, she developed delayed isolated ipsilateral sixth nerve palsy causing temporary lateral rectus weakness and diplopia. After 8 weeks, the palsy fully resolved spontaneously without any medical or surgical treatments11).
DFP is not an unusual complication following MVD and the incidence has already been reported by several authors to be about 2.5-8.3%10,14,15,20). These results were similar to those of our study, and we suggest the incidence of DFP to be 7.4% and all patients had either complete recovery (HB grade I) or minimal weakness (HB scale grade II). In our series, when we reviewed the characteristics of all patients and intraoperative findings, there did not seem to be any striking predisposing characteristics with which to predict the occurrence of this weakness except offending vessels. Offending vessels and herpes simplex virus (HSV) were significantly associated with DFP in our series (p=0.007, 0.005). A variety of offending vessels may cause difficulty in the surgery and contribute to nerve injury. But, the diagnosis of HSV was dependent on clinical symptoms such as watery blisters in the skin or mucous membrane of the mouth, lips. Serological test for specific HSV antibodies was not performed so there is insufficient evidence to conclude the association with HSV infection and DFP.
However, the etiology of delayed facial weakness is still unclear. Kim et al.10) reported 12 cases of DFP following MVD. They proposed that direct nerve trauma via Teflon felt or delayed facial nerve edema was associated with this complication. However in our cases, the nerve exit zone was probably not injured by the Teflon felt because several pieces of Teflon were carefully inserted without manipulation of the facial nerve and the occurrence of DFP was not associated with an increased number of Teflon felt pieces used during the operation.
Furukawa et al.6) and Franco-Vidal et al.5) suggested that HSV or varicella zoster virus reactivation is one of the mechanisms of delayed facial weakness. These possible diagnoses can be confirmed by serologic viral tests measuring antibody titers. In our series, 82 of 1354 patients were infected with herpes simplex virus following MVD, but none of them developed DFP.
However, we did not perform a serological search for specific herpes simplex virus antibodies or varicella zoster virus antibodies. Therefore, any viral etiology remains to be clarified.
Delayed hearing impairment is extremely rare after MVD. Kuchta et al.13) reported one case of delayed hearing loss, resulting in deafness after two weeks following MVD for trigeminal neuralgia. This patient did not recover from the hearing loss. The etiology of delayed hearing loss remains unclear. The author described that during the operation, the pia mater covering the brainstem and the cerebellum may be damaged. The eighth nerve may be unprotected, and susceptible to chemical and electrical insults.
Onoda et al.18) reported two cases of delayed hearing impairment. Two months after onset, the patients' hearing function improved dramatically. This result was different from the result of our case. There is controversy about whether patients with delayed hearing loss are able to make a complete recovery.
Sixth nerve palsy is not an uncommon complication in vascular lesions of the carotid artery and vertebral artery, in tumors or trauma of the clivus and cavernous sinus2,4,19). But, delayed isolated ipsilateral sixth nerve palsy following uncomplicated MVD for HFS is an extremely rare complication, and has not been reported before. In comparison with the facial nerve, the abducent nerve is long, thin, delicate, and more vulnerable to manipulation or intracranial pressure alterations. We suggest that even though the surgical procedure may have been uneventful, isolated ipsilateral sixth nerve palsy can occur.
In this large series we have demonstrated that the incidence of DFP is not as low as has been previously reported in the literature14,15,20). It does not have any striking predisposing factors. Delayed sixth nerve palsy and delayed hearing loss are extremely rare complications. Etiology of delayed cranial nerves palsies remains unclear. Delayed sixth, and seventh nerve palsies usually have good outcomes. In our experience, delayed hearing loss yields disappointing outcomes including incomplete recovery. There is controversy over the prognosis of delayed hearing loss compared with previous studies18). Further research is needed to clarify the etiology of delayed cranial nerve palsies.
References
1. Auger RG, Whisnant JP. Hemifacial spasm in Rochester and Olmsted County, Minnesota, 1960 to 1984. Arch Neurol. 1990; 47:1233–1234. PMID: 2241620.

2. Berlit P. Isolated and combined pareses of cranial nerves III, IV and VI. A retrospective study of 412 patients. J Neurol Sci. 1991; 103:10–15. PMID: 1865222.

3. Dallan I, De Vito A, Fattori B, Casani AP, Panicucci E, Berrettini S, et al. Intratympanic methylprednisolone in refractory sudden hearing loss : a 27-patient case series with univariate and multivariate analysis. Otol Neurotol. 2010; 31:25–30. PMID: 19940792.
4. De Ridder D, Menovsky T. Neurovascular compression of the abducent nerve causing abducent palsy treated by microvascular decompression. Case report. J Neurosurg. 2007; 107:1231–1234. PMID: 18077964.

5. Franco-Vidal V, Nguyen DQ, Guerin J, Darrouzet V. Delayed facial paralysis after vestibular schwannoma surgery : role of herpes viruses reactivation--our experience in eight cases. Otol Neurotol. 2004; 25:805–810. PMID: 15354015.

6. Furukawa K, Sakoh M, Kumon Y, Teraoka M, Ohta S, Ohue S, et al. [Delayed facial palsy after microvascular decompression for hemifacial spasm due to reactivation of varicella-zoster virus]. No Shinkei Geka. 2003; 31:899–902. PMID: 12968493.
7. Hanakita J, Kondo A. Serious complications of microvascular decompression operations for trigeminal neuralgia and hemifacial spasm. Neurosurgery. 1988; 22:348–352. PMID: 3352885.

8. Hitotsumatsu T, Matsushima T, Inoue T. Microvascular decompression for treatment of trigeminal neuralgia, hemifacial spasm, and glossopharyngeal neuralgia : three surgical approach variations : technical note. Neurosurgery. 2003; 53:1436–1441. discussion 1442-1443. PMID: 14633313.
9. Hyun SJ, Kong DS, Park K. Microvascular decompression for treating hemifacial spasm : lessons learned from a prospective study of 1,174 operations. Neurosurg Rev. 2010; 33:325–334. discussion 334. PMID: 20349099.

10. Kim BT, Hwang SC, Chang JC, Shin WH, Choi SK, Byun BJ. Delayed facial palsy following microvascular decompression in hemifacial spasm patients. J Korean Neurosurg Soc. 1999; 28:1332–1336.
11. Kim JW, Park K. Transient six nerve paresis with the delayed onset after microvascular decompression for hemifacial spasm. J Korean Skull Base Soc. 2007; 2:83–85.
12. Kong DS, Park K, Shin BG, Lee JA, Eum DO. Prognostic value of the lateral spread response for intraoperative electromyography monitoring of the facial musculature during microvascular decompression for hemifacial spasm. J Neurosurg. 2007; 106:384–387. PMID: 17367059.

13. Kuchta J, Møller AR, Wedekind C, Jannetta PJ. Delayed hearing loss after microvascular decompression of the trigeminal nerve. Acta Neurochir (Wien). 1998; 140:94–97. PMID: 9522915.

14. Kuroki A, Itagaki S, Nagai O. Delayed facial palsy after microvascular decompression for hemifacial spasm. Facial Nerve Res. 1991; 11:147–150.
15. Lovely TJ, Getch CC, Jannetta PJ. Delayed facial weakness after microvascular decompression of cranial nerve VII. Surg Neurol. 1998; 50:449–452. PMID: 9842870.

16. McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves : lessons learned after 4400 operations. J Neurosurg. 1999; 90:1–8. PMID: 10413149.

17. Olson S, Atkinson L, Weidmann M. Microvascular decompression for trigeminal neuralgia : recurrences and complications. J Clin Neurosci. 2005; 12:787–789. PMID: 16165362.

18. Onoda K, Ono S, Miyoshi Y, Tokunaga K, Date I. [Delayed hearing loss after microvascular decompression for hemifacial spasm : report of two cases]. No Shinkei Geka. 2006; 34:1045–1049. PMID: 17052017.
19. Patel SV, Mutyala S, Leske DA, Hodge DO, Holmes JM. Incidence, associations, and evaluation of sixth nerve palsy using a population-based method. Ophthalmology. 2004; 111:369–375. PMID: 15019392.

20. Rhee DJ, Kong DS, Park K, Lee JA. Frequency and prognosis of delayed facial palsy after microvascular decompression for hemifacial spasm. Acta Neurochir (Wien). 2006; 148:839–843. discussion 843. PMID: 16804640.

21. Santos-Lasaosa S, Pascual-Millán LF, Tejero-Juste C, Morales-Asín F. [Peripheral facial paralysis : etiology, diagnosis and treatment]. Rev Neurol. 2000; 30:1048–1053. PMID: 10904952.
22. She W, Dai Y, Du X, Yu C, Chen F, Wang J, et al. Hearing evaluation of intratympanic methylprednisolone perfusion for refractory sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2010; 142:266–271. PMID: 20115986.

23. Wang HB, Fan ZM, Han J, Li KY, Fan Z. [Serious complications of the microvascular decompression in cerebellopontine angle]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2005; 40:352–356. PMID: 16229176.
Fig. 1
The onset time for delayed facial palsy averaged 11.2 days with a range between 2 to 23 days. DFP : delayed facial palsy.

Fig. 3
Three weeks after MVD, PTA-SA shows scale-out on the operated side. MVD : microvascular decompression, PTA-SA : pure tone audiometry and speech discrimination.

Fig. 4
One month after onset, PTA-SA shows ipsilateral hearing function improved. PTA-SA : pure tone audiometry and speech discrimination.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download