Abstract
Objective
To evaluate the effect of calcium supplementation on spinal bone fusion in ovariectomized (OVX) rats.
Methods
Sixteen female Sprague Dawley rats underwent bilateral ovariectomy at 12 weeks of age to induce osteoporosis and were randomly assigned to two groups : control group (n=8) and calcium-supplemented group (OVX-Ca, n=8). Autologous spinal bone fusion surgery was performed on both groups 8 weeks later. After fusion surgery, the OVX-Ca group was supplemented with calcium in drinking water for 8 weeks. Blood was obtained 4 and 8 weeks after fusion surgery. Eight weeks after fusion surgery, the rats were euthanized and the L4-5 spine removed. Bone fusion status and fusion volume were evaluated by manual palpation and three-dimensional computed tomography.
Results
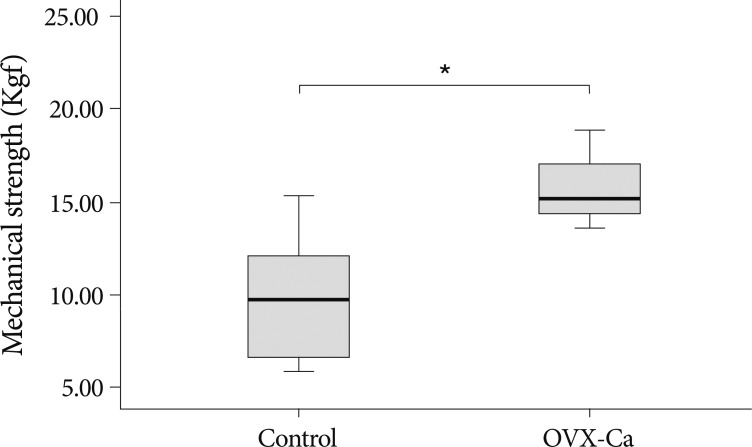
The mean fusion volume in the L4-5 spine was significantly greater in the OVX-Ca group (71.80±8.06 mm3) than in controls (35.34±8.24 mm3) (p<0.01). The level of osteocalcin, a bone formation marker, was higher in OVX-Ca rats than in controls 4 weeks (610.08±10.41 vs. 551.61±12.34 ng/mL) and 8 weeks (552.05±19.67 vs. 502.98±22.76 ng/mL) after fusion surgery (p<0.05). The level of C-terminal telopeptide fragment of type I collagen, a bone resorption marker, was significantly lower in OVX-Ca rats than in controls 4 weeks (77.07±12.57 vs. 101.75±7.20 ng/mL) and 8 weeks (69.58±2.45 vs. 77.15±4.10 ng/mL) after fusion surgery (p<0.05). A mechanical strength test showed that the L4-5 vertebrae in the OVX-Ca group withstood a 50% higher maximal load compared with the controls (p<0.01).
Osteoporosis is a bone metabolic disease characterized by low bone mineral density, which leads to bone fragility and an increased risk of fracture35). Osteoporosis causes a difficult environment for spinal bone fusion surgery in patients with a degenerative spinal disease or traumatic fracture9,19,21,40). In senile or postmenopausal osteoporosis patients, spinal bone fusion surgery is associated with complications such as nonunion, malunion, pseudarthrosis, and instrument failure5,39,42). Due to these complications, alternative treatment or improvements with surgical technique are currently under investigation.
Recently, a large number of osteoporotic patients with bone fragility have been treated with antiresorptive drugs such as bisphosphonates and selective estrogen receptor modulators after spinal arthrodesis18,23,29,38). However, there exists debate about the actual effects of these drugs24,31), and their use is limited to patients with osteopenia or underestimated osteoporosis due to bony spur or calcified structures around bone. Recent studies have reported on the positive effect of calcium supplementation on fracture healing of osteoporotic bone in ovariectomized rats6,20,37). Calcium is required for normal skeletal growth and mineralization, and plays an important role in regulating bone remodeling and bone mass30,32). However, to our knowledge, no controlled studies have been undertaken to determine whether increased calcium intake promotes bone fusion volume and mechanical strength in spinal fusion surgery.
We evaluated the effect of calcium supplementation on spinal bone fusion in ovariectomized rats. We measured the fusion volume using three-dimensional microcomputed tomography (3D-µCT), the levels of biochemical bone metabolism markers [osteocalcin, C-terminal telopeptide fragment of type I collagen (CTX)], and bone mechanical strength in ovariectomized rats.
All animal experiments were performed in accordance with the National Institute of Health guidelines on animal care and were approved by the Institutional Animal Care Committee. Sixteen female Sprague Dawley rats aged 11 weeks were purchased from Samtako Bio Inc. (Osan, Korea) and acclimated to the laboratory conditions for 1 week before the experiment. The rats were housed in an air-conditioned room with a 12-hrs light/dark cycle at a room temperature of 22±2℃ and humidity of 45-65%, and given free access to food and tap water. At 12 weeks of age, they underwent bilateral ovariectomy to induce osteoporosis. We used the double dorsolateral approach described in detail by Park et al.34). Initially, the rats were anesthetized intraperitoneally with a mixture of xylazine (10 mg/kg) and ketamine (60 mg/kg). After bilateral skin incision just medial to the most bulging part of the back, the peritoneal cavity was entered by dissecting through the muscles to reveal the adipose tissue surrounding the ovary. After the surgery, muscle, fascia, and skin were sutured using silk sutures.
Throughout the experimental period, the body weight was monitored once a week. Blood samples were obtained by cardiac puncture at baseline, 8 weeks after the ovariectomy, and 4 and 8 weeks after spinal bone fusion surgery (represented in Fig. 1 as 8+4 and 8+8 weeks). Serum was separated by centrifugation at 1500×g and stored at -80℃ for later measurement of bone metabolic marker levels.
The entire experimental schedule is represented in Fig. 1.
Anesthesia was induced with ketamine intraperitoneally (90 mg/kg i.p.). After the surgical site was shaved and prepared, the rat was placed prone on the operating table. An L4-5 posterolateral intertransverse and translaminar arthrodesis was performed as described by Abe et al.1). Briefly, a posterior midline incision was made over the lumbar spine. The laminar-transverse space of the L4 and L5 vertebrae was exposed by splitting the back muscles (modified Wiltse approach). Once exposed, the laminar-transverse space of the L4-5 vertebrae was decorticated with an electric burr until a blush of cancellous bone was observed. About 0.3 g of autologous bone was harvested from both iliac crests through fascial incisions. The harvested iliac bone was morselized and implanted bilaterally on the decorticated fusion beds to bridge the L4 and L5 interlaminar and intertransverse space. Postoperative antibiotics were given subcutaneously (gentamicin, 0.5 mg/kg).
After fusion surgery, the rats were divided into two groups : a control group and a calcium-supplemented group (OVX-Ca group). Calcium was supplemented by mixing 1% lactic acid hemicalcium salt (Sigma, St. Louis, MO, USA) in the drinking water, which was provided ad libitum. This method of calcium supplementation does not stress the rats compared with oral gavage or intramuscular injection and has been shown to be optimal for bone growth37). We measured serum calcium concentration to ensure adequate calcium intake.
Eight weeks after fusion surgery, the rats were sacrificed and the L4-5 segments were removed. Fusion at the bone-grafted segment was assessed by manual palpation10,22) and 3D-µCT scanning at the L4-5 segment, as described by Abe et al.1). Briefly, the harvested lumbar spine was palpated gently, and lateral side-bending motion at the L4-5 level was compared with the motion at the adjacent levels above (L3-4) and below (L5-6). Two independent neurosurgeons who were blinded to the grouping of animals tested the stability of lateral side-bending motion at the operative spinal segment.
µCT scanning (eXplore Locus SP, GE Healthcare, USA) of the lumber spine was performed under consistent conditions. The formation of bridging bone between the L4-5 laminar-transverse space and consolidation of the grafted bone were evaluated. Based on the results of manual palpation testing and 3D-µCT scanning, each specimen was classified as a solid union when no motion was observed by the two neurosurgeons and bony continuity between the L4 and L5 laminar-transverse vertebrae was observed and as a nonunion when motion was detected or discontinuity was observed22,41).
Bone morphometric parameters of the fusion mass in the L4 and L5 vertebrae were assessed using µCT. The scanning protocol was set at X-ray energy settings of 80 kV and 80 µA, and the sample was scanned over one entire 360° rotation with an exposure time of 3000 ms/frame. An isotopic resolution of 15-40 µm voxel size that displayed the microstructure of the rat lumbar vertebrae was selected, and the angle of increment around the sample was set to 0.40, which resulted in the acquisition of 900 two-dimensional (2D) images. A modified Feldkamp cone-beam algorithm was used to reconstruct the 2D projections into a 3D volume.
For the bone analysis, bone tissue from the region of the fusion mass in the L4-5 vertebrae was selected as the region of interest, and image information was obtained based on the automatic domain value yielded by the computer. Bone volume fraction was applied to perform quantitative analysis using software provided with the 2.0+ ABA Microview of the µCT system.
The serum level of osteocalcin, a sensitive biomarker of bone formation, was estimated using an osteocalcin EIA kit (Biomedical Technologies, Stoughton, MA, USA). The effects of the treatments on bone resorption were evaluated using a RatLaps ELISA kit (Nordic Bioscience Diagnostics, Herlev, Denmark) to detect CTX generated by osteoclasts.
Mechanical spinal strength was assessed using a three-point bending test2,13). Each bone was positioned on the two lower supports of the anvil of a Universal Testing Machine (Instron 4202; Instron, Canton, MA, USA). Load was applied to the midportion of the fusion mass in the L4-5 vertebrae using a crosshead speed of 1.5 mm/min for all tests. The load versus displacement data were recorded automatically by the Instron software (INSTRON series IX Automated Materials Tester, version 8.04.00), which calculates the mechanical parameters from the load-displacement curves.
All statistical comparisons were computed using SPSS 17.0 software. The data were expressed as mean±standard deviation. Repeated measure analysis of variance was used to compare body weights between the two groups. A two-sample t test was used to identify differences between the groups. A p value <0.05 was considered significant.
Body weight was measured once a week throughout the 16-week experimental period. Changes in the mean body weights between the two groups over time are illustrated in Table 1. Body weight did not differ between two groups throughout the experimental period.
The serum calcium concentrations in both groups are presented in Table 2. The serum calcium concentration was significantly higher in the OVX-Ca group than in the control group at both 4 and 8 weeks after spinal bone fusion surgery (p<0.05). This shows that the intake of calcium was appropriate in the OVX-Ca group.
The manual palpation testing showed that the fusion rates, defined as the percentage of solid union, at 8 weeks after fusion surgery were 85% in the OVX-Ca group and 42% in the control group, although this difference was not significant (p>0.05). The 3D-µCT scanning indicated that the OVX-Ca group had a greater and denser fusion mass compared with the control group 8 weeks after fusion surgery. This was a significant different fusion rate between the two groups (OVX-Ca : 85%, control : 28%; p<0.05).
Eight weeks after fusion surgery, the fusion masses comprised a mixture of dispersed grafted bone fragments and newly formed bone in the OVX-Ca group. The fusion volume differed between the two groups (p<0.01) (Fig. 2). At that time, the fusion masses had matured with the development of a cortical shell around the trabecular bone (Fig. 3).
The serum osteocalcin level in the OVX-Ca group remained high 4 and 8 weeks after fusion surgery. By contrast, the osteocalcin level decreased gradually in the control group from 4 to 8 weeks after fusion surgery. The serum osteocalcin level was higher in the OVX-Ca group than in the control group 4 and 8 weeks after fusion surgery (p<0.05) (Fig. 4A).
The serum CTX levels increased in both the OVX-Ca and control groups in the first 4 weeks after bone fusion surgery but had decreased by 8 weeks after fusion surgery. Although the CTX level increased in both groups in the first 4 weeks after bone fusion surgery, the CTX level remained significantly lower in the OVX-Ca group than in the control group throughout the postoperative course (p<0.05) (Fig. 4B).
Biomechanical testing was performed to measure the strength of the fused bone mass. The two groups had similar stress values. The three-point bending test showed that the fused vertebrae in the OVX-Ca group could withstand a 50% greater maximal load compared with the control group (p<0.01) (Fig. 5).
After menopause, depletion of estrogen increases bone turnover, and the rate of osteoclastic resorption exceeds the rate of osteoblastic formation, resulting in a loss of bone mass8). Based on this observation, patients with osteoporosis and osteopenia may not be good candidates for fusion surgery due to instrument or fusion failure following surgery for traumatic fracture and degenerative spinal disease. It is estimated that more than 200000 spine fusion procedures are performed each year in the United States3). Posterior lumbar arthrodesis is the most common procedure performed, and failure to achieve a solid bony union (nonunion) occurs in 10-40% of patients with a single-level fusion and more frequently when multiple levels are attempted11,12,27).
Various kinds of drugs used in the treatment of osteoporosis, such as bisphosphonates, parathyroid hormone, estrogen, selective estrogen receptor modulators, calcitonin, and vitamin D, are options for inducing osteoporotic fracture healing14,17,20,29,36,37). However, whether these drugs are effectively help the patients with osteoporosis or osteopenia is unclear. Lehman et al.28) reported that alendronate sodium inhibited or delayed bone fusion after intertransverse process spinal fusion in a rabbit model. Presumably, this resulted from uncoupling of the balance between osteoclastic and osteoblastic activities inherent in bone healing. By contrast, Nagahama et al.31) found that favorable mechanical circumstances provided by alendronate overcame its detrimental biological effects on the healing process after spinal fusion. Therefore, they recommended that osteoporosis patients undergoing spinal fusion take bisphosphonates throughout the postoperative period. In addition, at low intermittent pulsatile doses, parathyroid hormone administration increases bone formation in rats7,26,33). However, the clinical use of parathyroid hormone has several disadvantages, such as the need for daily subcutaneous injection in the abdomen or thigh, and the expense relative to other drugs4).
Calcium is one of the most important nutrients in the healing of bone fractures30). Some studies have reported on the structural changes induced by calcium treatment during fracture healing6,15,19,37). However, there is still lack of knowledge about the effects of calcium on spinal bone fusion with osteoporosis. Thus, we examined the effect of calcium supplementation on spinal bone fusion in the ovariectomized rat. In the present study, 3D-µCT images showed significant changes in the structural characteristics of the fusion mass in animals treated with calcium. Calcium-treated animals had significantly more abundant fusion volumes compared with the control animals. Calcium intake increases bone formation and decreases bone resorption activity at the graft site, which seems to have beneficial effects on graft bone healing, especially in bone remodeling after new bone formation30). In our study, the serum level of osteocalcin, an osteoblast marker, peaked 4 weeks after surgery in the OVX-Ca rats and was higher at 4 and 8 weeks after surgery in OVX-Ca group than in the control group (Fig. 4A) (p<0.05). This finding suggests that calcium intake has positive effects on osteoblast activation after fusion surgery in the osteoporotic condition. The level of serum CTX, a bone resorption marker, increased in both groups, peaked 4 weeks after surgery, and then decreased from 4 to 8 weeks after surgery. Serum CTX level was lower in the OVX-Ca group than in the control group throughout the postoperative course (Fig. 4B) (p<0.05). These findings propose that calcium agents shifted the bone formation/resorption balance in a positive direction during the bone graft fusion process.
These results are consistent with previous findings in experimental osteoporotic-fracture models6,15,21,25,37). Ahmad et al.37) studied the effects of calcium supplementation on fracture healing of osteoporotic bone in ovariectomized rats. Calcium supplements appeared to improve fracture healing of osteoporotic bone. Half of the ovariectomized rats were given calcium supplements throughout the healing phases, and this may explain the similar radiological evidence of fracture healing in the supplemented and sham-operated rats.
In ovariectomized rats, compared to the Sham group (non-operated rats), serum ionized-calcium levels showed no significant differences in other experimental study16). Therefore, we did not compare the sham group with the calcium supplementated group. In this study, after fusion surgery, the serum calcium concentration was significantly higher in the OVX-Ca group than in the control group for postoperative period (p<0.05).
Mechanical strength of the healed fractured bone is a reliable test of the bone's recovery to its normal strength2,13). In our study, we used the three-point bending test. The fused vertebrae in the OVX-Ca group could withstand a 50% higher maximal load compared with the control group (Fig. 5) (p<0.01). These results suggest that calcium intake can increase the mechanical strength by increasing the fusion mass volume. These positive effects on bone formation correspond to those of other studies showing that drugs used to treat osteoporosis augment spinal fusion mass volume in experimental models14,33,38). However, we did not perform pathological examination of the bone mass, and this finding does not necessarily indicate an improvement in the fusion mass quality. The fusion mass quality should be evaluated in future studies.
In the present study, we attempted to answer the key question about the relationship between calcium intake and spinal fusion in osteoporosis : Specifically, is the addition of dietary calcium sufficient to provide a clinical advantage for patients undergoing spinal fusion in osteoporosis? Our findings suggest that calcium supplementation improves mechanical strength and that this may overcome its complicated biological effect on the healing process in spinal fusion. We recommend that osteoporosis patients undergoing spinal fusion should receive an adequate intake of calcium throughout the perioperative period.
Finally, several questions remain to be answered in future research. 1) For how long should patients take calcium before and after spinal fusion surgery to improve the strength of vertebral bodies? 2) What is the optimal dose of calcium to increase the bone fusion mass? 3) Does the bridging bone formed during calcium treatment lead to superior bone quality? These questions will form the basis of our future studies.
References
1. Abe Y, Takahata M, Ito M, Irie K, Abumi K, Minami A. Enhancement of graft bone healing by intermittent administration of human parathyroid hormone (1-34) in a rat spinal arthrodesis model. Bone. 2007; 41:775–785. PMID: 17707711.

2. Balena R, Toolan BC, Shea M, Markatos A, Myers ER, Lee SC, et al. The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. J Clin Invest. 1993; 92:2577–2586. PMID: 8254015.

3. Boden SD. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine (Phila Pa 1976). 2002; 27:S26–S31. PMID: 12205416.

4. Borgström F, Ström O, Marin F, Kutahov A, Ljunggren O. Cost effectiveness of teriparatide and PTH(1-84) in the treatment of postmenopausal osteoporosis. J Med Econ. 2010; 13:381–392. PMID: 20604678.

5. Bridwell KH, Sedgewick TA, O'Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 1993; 6:461–472. PMID: 8130395.

6. Chen H, Hayakawa D, Emura S, Ozawa Y, Okumura T, Shoumura S. Effect of low or high dietary calcium on the morphology of the rat femur. Histol Histopathol. 2002; 17:1129–1135. PMID: 12371141.
7. Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis : a paired biopsy study. J Bone Miner Res. 2001; 16:1846–1853. PMID: 11585349.

9. Diamond TH, Clark WA, Kumar SV. Histomorphometric analysis of fracture healing cascade in acute osteoporotic vertebral body fractures. Bone. 2007; 40:775–780. PMID: 17141596.

10. Erulkar JS, Grauer JN, Patel TC, Panjabi MM. Flexibility analysis of posterolateral fusions in a New Zealand white rabbit model. Spine (Phila Pa 1976). 2001; 26:1125–1130. PMID: 11413423.

11. Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, Kurz LT. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976). 1997; 22:2807–2812. PMID: 9431616.
12. France JC, Yaszemski MJ, Lauerman WC, Cain JE, Glover JM, Lawson KJ, et al. A randomized prospective study of posterolateral lumbar fusion. Outcomes with and without pedicle screw instrumentation. Spine (Phila Pa 1976). 1999; 24:553–560. PMID: 10101819.
13. French DL, Muir JM, Webber CE. The ovariectomized, mature rat model of postmenopausal osteoporosis : an assessment of the bone sparing effects of curcumin. Phytomedicine. 2008; 15:1069–1078. PMID: 18693096.

14. Fu L, Tang T, Miao Y, Hao Y, Dai K. Effect of 1,25-dihydroxy vitamin D3 on fracture healing and bone remodeling in ovariectomized rat femora. Bone. 2009; 44:893–898. PMID: 19442605.

15. Gala J, Díaz-Curiel M, de la Piedra C, Calero J. Short- and long-term effects of calcium and exercise on bone mineral density in ovariectomized rats. Br J Nutr. 2001; 86:521–527. PMID: 11591240.

16. García-Contreras F, Paniagua R, Avila-Díaz M, Cabrera-Muñoz L, Martínez-Muñiz I, Foyo-Niembro E, et al. Cola beverage consumption induces bone mineralization reduction in ovariectomized rats. Arch Med Res. 2000; 31:360–365. PMID: 11068076.

17. Gasser JA, Ingold P, Venturiere A, Shen V, Green JR. Long-term protective effects of zoledronic acid on cancellous and cortical bone in the ovariectomized rat. J Bone Miner Res. 2008; 23:544–551. PMID: 18072878.

18. Gezici AR, Ergün R, Gürel K, Yilmaz F, Okay O, Bozdoğan O. The effect of risedronate on posterior lateral spinal fusion in a rat model. J Korean Neurosurg Soc. 2009; 46:45–51. PMID: 19707493.

19. Giannoudis P, Tzioupis C, Almalki T, Buckley R. Fracture healing in osteoporotic fractures : is it really different? A basic science perspective. Injury. 2007; 38(Suppl 1):S90–S99. PMID: 17383490.
20. Grases F, Sanchis P, Prieto RM, Perelló J, López-González ÁA. Effect of tetracalcium dimagnesium phytate on bone characteristics in ovariectomized rats. J Med Food. 2010; 13:1301–1306. PMID: 21091244.

21. Hao YJ, Zhang G, Wang YS, Qin L, Hung WY, Leung K, et al. Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone. 2007; 41:631–638. PMID: 17652051.

22. Hidaka C, Goshi K, Rawlins B, Boachie-Adjei O, Crystal RG. Enhancement of spine fusion using combined gene therapy and tissue engineering BMP-7-expressing bone marrow cells and allograft bone. Spine (Phila Pa 1976). 2003; 28:2049–2057. PMID: 14501913.

23. Honig S. Osteoporosis -- new treatments and updates. Bull NYU Hosp Jt Dis. 2010; 68:166–170. PMID: 20969546.
24. Huang RC, Khan SN, Sandhu HS, Metzl JA, Cammisa FP Jr, Zheng F, et al. Alendronate inhibits spine fusion in a rat model. Spine (Phila Pa 1976). 2005; 30:2516–2522. PMID: 16284589.

25. Kasukawa Y, Miyakoshi N, Maekawa S, Nozaka K, Noguchi H, Shimada Y. Effects of alfacalcidol on muscle strength, muscle fatigue, and bone mineral density in normal and ovariectomized rats. Biomed Res. 2010; 31:273–279. PMID: 21079356.

26. Kitazawa R, Imai Y, Fukase M, Fujita T. Effects of continuous infusion of parathyroid hormone and parathyroid hormone-related peptide on rat bone in vivo : comparative study by histomorphometry. Bone Miner. 1991; 12:157–166. PMID: 2021707.

27. Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis : a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976). 2004; 29:726–733. discussion 733-734. PMID: 15087793.
28. Lehman RA Jr, Kuklo TR, Freedman BA, Cowart JR, Mense MG, Riew KD. The effect of alendronate sodium on spinal fusion : a rabbit model. Spine J. 2004; 4:36–43. PMID: 14749192.
29. Li YF, Zhou CC, Li JH, Luo E, Zhu SS, Feng G, et al. The effects of combined human parathyroid hormone (1-34) and zoledronic acid treatment on fracture healing in osteoporotic rats. Osteoporos Int. 2012; 23:1463–1474. PMID: 21892678.

30. Matkovic V, Heaney RP. Calcium balance during human growth : evidence for threshold behavior. Am J Clin Nutr. 1992; 55:992–996. PMID: 1570810.
31. Nagahama K, Kanayama M, Togawa D, Hashimoto T, Minami A. Does alendronate disturb the healing process of posterior lumbar interbody fusion? A prospective randomized trial. J Neurosurg Spine. 2011; 14:500–507. PMID: 21275549.

32. Nordin BE, Heaney RP. Calcium supplementation of the diet : justified by present evidence. BMJ. 1990; 300:1056–1060. PMID: 2188698.

33. O'Loughlin PF, Cunningham ME, Bukata SV, Tomin E, Poynton AR, Doty SB, et al. Parathyroid hormone (1-34) augments spinal fusion, fusion mass volume, and fusion mass quality in a rabbit spinal fusion model. Spine (Phila Pa 1976). 2009; 34:121–130. PMID: 19112335.
34. Park SB, Lee YJ, Chung CK. Bone mineral density changes after ovariectomy in rats as an osteopenic model : stepwise description of double dorso-lateral approach. J Korean Neurosurg Soc. 2010; 48:309–312. PMID: 21113356.

35. Proceedings of a symposium. Consensus Development Conference on Osteoporosis. October 19-20, 1990, Copenhagen, Denmark. Am J Med. 1991; 91:1S–68S.
36. Shiraishi A, Miyabe S, Nakano T, Umakoshi Y, Ito M, Mihara M. The combination therapy with alfacalcidol and risedronate improves the mechanical property in lumbar spine by affecting the material properties in an ovariectomized rat model of osteoporosis. BMC Musculoskelet Disord. 2009; 10:66. PMID: 19527501.

37. Shuid AN, Mohamad S, Mohamed N, Fadzilah FM, Mokhtar SA, Abdullah S, et al. Effects of calcium supplements on fracture healing in a rat osteoporotic model. J Orthop Res. 2010; 28:1651–1656. PMID: 20572125.

38. Takahata M, Ito M, Abe Y, Abumi K, Minami A. The effect of anti-resorptive therapies on bone graft healing in an ovariectomized rat spinal arthrodesis model. Bone. 2008; 43:1057–1066. PMID: 18835375.

39. West JL 3rd, Bradford DS, Ogilvie JW. Results of spinal arthrodesis with pedicle screw-plate fixation. J Bone Joint Surg Am. 1991; 73:1179–1184. PMID: 1890118.

40. Xu SW, Wang JW, Li W, Wang Y, Zhao GF. [Osteoporosis impairs fracture healing of tibia in a rat osteoporotic model]. Zhonghua Yi Xue Za Zhi. 2004; 84:1205–1209. PMID: 15387984.
41. Yee AJ, Bae HW, Friess D, Robbin M, Johnstone B, Yoo JU. Accuracy and interobserver agreement for determinations of rabbit posterolateral spinal fusion. Spine (Phila Pa 1976). 2004; 29:1308–1313. PMID: 15187630.

42. Zdeblick TA. A prospective, randomized study of lumbar fusion. Preliminary results. Spine (Phila Pa 1976). 1993; 18:983–991. PMID: 8367786.
Fig. 1
Experimental groups and schedule. Sixteen female Sprague Dawley rats underwent bilateral ovariectomy at 12 weeks of age to induce osteoporosis. Then, autologous spinal bone fusion surgery was performed on both groups (Control; n=8, OVX-Ca; n=8) 8 weeks later. Eight weeks after fusion surgery, the rats were euthanized and the L4-5 spine was removed. The serum concentrations of markers of bone metabolism were measured by ELISA at baseline, 8 weeks after ovariectomy, and 4 and 8 weeks after fusion surgery. The bone fusion status and fusion volume were evaluated by manual palpation and 3D-µCT scanning. The mechanical strength was also tested. OVX-Ca : calcium-supplemented group.

Fig. 2
Fusion volume. At 8 weeks after fusion surgery, the fusion masses comprised a mixture of dispersed grafted bone fragments and newly formed bone in the OVX-Ca group (p<0.01). *p<0.01 for OVX-Ca vs. control group. OVX-Ca : calcium-supplemented group.

Fig. 3
Representative µCT images of vertebrae from the two groups. OVX-Ca rats show larger fusion mass than controls eight weeks after surgery. ▸ : fusion mass, OVX-Ca : calcium-supplemented group.
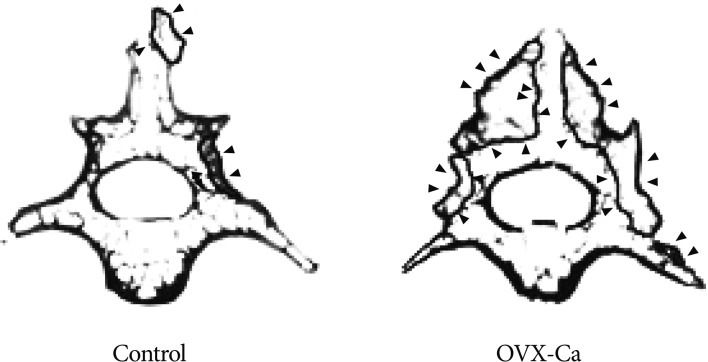
Fig. 4
Serum osteocalcin concentration (A) as a marker of bone formation. The concentration of C-terminal telopeptide fragment of type I collagen (CTX-1) (B) was used as a marker of bone resorption. Values are represented as the mean±standard deviation. *p<0.05 for the OVX-Ca vs. control group. OVX-Ca : calcium-supplemented group.

Fig. 5
The loading force to the maximal load on the fusion mass in L4 and L5 vertebrae determined using a three-point bending test. The OVX-Ca group significantly increases the maximal load as compared with to the control group (p<0.01). *p<0.01 for OVX vs. control group comparison. OVX-Ca : calcium-supplemented group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download