Abstract
Metronidazole-induced encephalopathy is a very rare complication of the long standing use of metronidazole. The encephalopathy is bilateral and symmetric in nature. We report on the magnetic resonance imaging (MRI) and clinical course of metronidazole-induced encephalopathy in a 60-year-old female with a persistent anaerobic brain abscess after draining of the abscess. After 3 months of metronidazole administration, the patient complained of dysarthria, tingling sense of all extremities, and left hemiparesis. MRI revealed symmetric hyperintensity lesions in medulla, pons, dentate nuclei of cerebellum, and splenium of corpus callosum, all of which represent typical findings of metronidazole-induced encephalopathy. In addition, asymmetric lesions in midbrain, thalamus, putamen and cerebral subcortical white matter were noted. The patient recovered after discontinuation of metronidazole and the remaining abscess was successfully treated with meropenem and levofloxacine.
Metronidazole-induced encephalopathy (MIE) is a rare clinical condition resulting from long-term use of metronidazole. Typical findings include progressive clinical deterioration such as dysarthria, gait disturbance, sensory changes in the extremities, confusion, nausea, vomiting, and dysmetria with multiple signal changes in cerebellar dentate nuclei, corpus callosum, midbrain, and cerebral white matter1,2,8,11,12,14,16). The conditions tend to be reversed when drug use is stopped1,2,8,11,12,14,16). All the lesions associated with MIE have been reported as symmetric and this symmetric nature has been regarded as a key point of MIE. Presently, we describe magnetic resonance imaging (MRI) findings and clinical course of MIE with asymmetric involvement of unilateral cerebral peduncle, thalamus, putamen and cerebral subcortical white matter, in addition to typical symmetric lesions in medulla, pons, dentate nuclei of cerebellum, and splenium of corpus callosum.
A 60-year-old female who underwent a dental treatment 2 weeks before hospitalization, visited the hospital with the chief complaint of a headache. The patient had no previous history of alcohol abuse or malnutrition. Two years previously, the patient had been diagnosed with liver cirrhosis caused by chronic hepatitis B, and had undergone two cycles of peg interferon α-2β therapy. The patient did not have any focal neurological symptoms during the neurological examination. Brain computed tomography (CT) revealed the presence of a brain abscess of about 30 mL on the left frontal lobe (Fig. 1). The abscess was drained by stereotactic catheter insertion. Culture examination identified Streptococcus sanguis, Peptostreptococcus, and Bacteroides species. Based on the verified culture test, the brain abscess was assumed to be caused by the invasion of normal anaerobic flora in the oral cavity. Subsequently, metronidazole was administered intravenously. After the administration of 15 g of metronidazole over a period of 10 days, there was a decrease in the size of the abscess, but neutropenia was prominent in a blood test (white blood cell count 1800/uL and absolute neutrophil count 300/uL). Under the impression of drug-induced neutropenia, administration of metronidazole was discontinued and meropenem was substituted as a new antibiotic. One week later, deterioration in the patient's mental state was observed. A CT scan revealed a marked increase in the size of the abscess. A second stereotactic aspiration was carried out and metronidazole was re-administered. The patient soon recovered to her normal neurologic status. Subsequently, serial CT follow-ups were carried out and a decrease in the size of the abscess was noted. However, there was no ultimate disappearance of the abscess. Hence, administration of metronidazole was continued for the next 3 months.
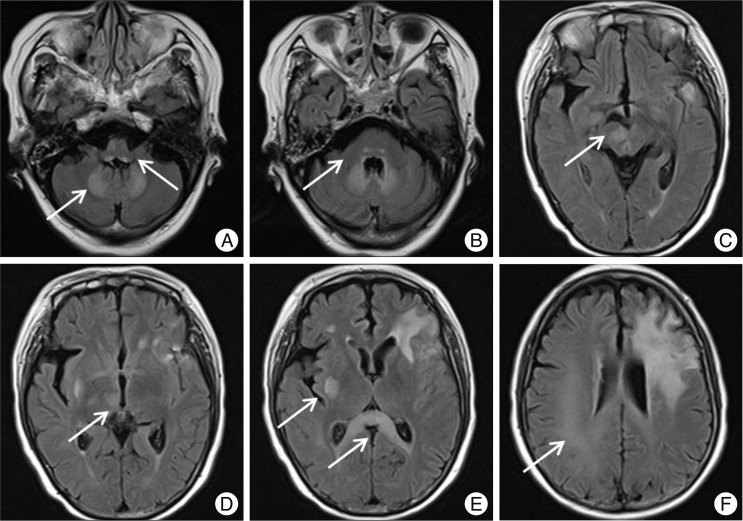
After the re-administration of metronidazole (1.5 g per day, total dosage, 156.5 g) for 11 weeks, the patient complained of subtle changes in sensation and subjective weakness on the left lower extremity. The patient's mood became depressive and nervous. Brain CT, spinal MRI, and nerve conduction velocity were checked without any remarkable findings, except for the small remnant of abscess. During the next week of closed observation, progression of tingling sensation on the left leg was apparent, which slowly extended to the ipsilateral upper extremity. The patient also had slightly ataxic gait. On day 84 subsequent to readministration of metronidazole, development of dysarthria and weakness on the left side with motor power of grade 3 were noted. Emergent brain MRI showed no recurrence of brain abscess, but a symmetric, round-shaped, high-signal intensity was found at the dorsal medullae, dentate nuclei (Fig. 2A), and pons (Fig. 2B) on T2-weighted image and fluid attenuated inversion recovery (FLAIR) imaging. The corpus callosum was also symmetrically involved along with the splenium (Fig. 2E). In addition, there were multiple asymmetric lesions in the midbrain (Fig. 2C), thalamus (Fig. 2D), putamen (Fig. 2E), and subcortical white matter on right side of the brain (Fig. 2F). Among these lesions, lesions in the splenium of the corpus callosum, midbrain, and cerebral subcortical white matter were of higher intensity on diffusion weighted imaging (DWI). In the subcortical white matter, low signal on apparent diffusion coefficient (ADC) map was observed at the corresponding areas of high DWI.
MIE was strongly suspected based on the clinical and imaging findings, and administration of metronidazole was promptly discontinued. After discontinuation of metronidazole, the patient progressively recovered from MIE-related symptoms. Hemiparesis was the first symptom to be relieved after one week of discontinuation of the drug, dysarthria after 2 weeks and paresthesia after 4 weeks. A combined regimen of meropenem and levofloxacin was administrated for observational periods after discontinuation of metronidazole.
Follow-up FLAIR MRI after 4 weeks of the new antibiotic regimen showed normalization of abnormal lesions and disappearance of the remaining brain abscess. However, abnormal high signal intensity in the splenium of the corpus callosum was persistent even though previous symptoms related to MIE had all disappeared. The patient has been symptom-free without recurrence of brain abscess for two-year follow-up.
Metronidazole, the prototype nitroimidazole antimicrobial, is bactericidal in nature because of its metabolites, which cause DNA strand breakage in anaerobic and protozoal microbes13). Metronidazole attains 60-100% of plasma concentrations in most tissues, including the central nervous system13), so it is a drug of choice for the treatment of anaerobic brain abscess. The side effects include peripheral neuropathy, convulsive seizures, encephalopathy, ataxic gait, and dysarthria1,11,14-16). Among the side effects, MIE has been very rarely reported1,2,8,11,14-16). The mechanisms that underlie metronidazole's neuronal toxicity include the oxidation of catecholamine neurotransmitters (such as norepinephrine, dopamine, and related catecholamine derivatives) to form semiquinone radicals and nitro anion radicals, which reduce tissue oxygen and generate the superoxide radical and hydrogen peroxide in a reaction with 5-nitroimidazole derivatives, such as metronidazole15). These radicals are proposed to cause neurotoxicity in the course of metronidazole treatment15). The total dosage of metronidazole and duration for medication resulting in MIE has been reported as 45-120 g14) and 1-12 weeks1,2,14), respectively. In our case, total dosage exceeded 156.5 g over a 3-month period of use. Long-term administration of high doses of metronidazole might influence the clinical and radiological severity of MIE. However, the quantitative relationship between metronidazole dosage and severity of MIE is unknown. Interestingly, over half of the total worldwide cases reported have occurred in Koreans2,11,12,14,16). This finding may reflect endemic prevalence of infectious organisms, which are susceptible to metronidazole administration.
MIE is a disease that can be diagnosed purely based on the clinical history and imaging findings. MIE has two distinctive features that make it readily discernable from other metabolic diseases : patients always have a history of long-term use of metronidazole and the symptoms and lesions apparent on MRI can be reversed after discontinuation of metronidazole usage1,2,8,11,14-16). Radiological findings of MIE have been reported in terms of the presence of bilateral symmetric high signal intensities on FLAIR or T2-weighted image in cerebellar dentate nucleus1,2,8,12,14), midbrain12), corpus callosum1,2,8,12,14), dorsal pons2,12,14), medulla2,8,12,14), periaqueductal region8) and cerebral white matter1,2,11,12,14). Symmetrical involvement is nearly always present; an exception is the recent report of MIE involving asymmetric subcortical white matter2). The present case provides another example. In our case, asymmetric lesions were found not only in subcortical white matter but also in midbrain, thalamus, and putamen. Furthermore, to the best of our knowledge, this is the first report of MIE with an involvement of thalamus. Despite of the lack of pathologic confirmation, our case could be easily differentiated from other condition such as demyelinating, metabolic, and infectious diseases. Wernicke encephalopathy and Marchiafava-Bignami disease are primarily associated with malnutrition and chronic alcoholism. With respect to differential diagnosis, Wernicke encephalopathy tends to show a predilection for the midbrain and diencephalon11) and Marchiafava-Bignami disease tends to invade from the body into the genu and splenium of the corpus callosum14). These findings differ from MIE in which the deep cerebellar nuclei are likely to be most sensitive to the effects of metronidazole toxicity and are the most specific observed imaging manifestation8) followed by involvement of subcortical white matter, splenium of courpus callosum and mesencephalon1-4,6,8-12,14,16,17). Viral encephalitis, osmotic demyelination syndrome, variant Creutzfeldt-Jakob disease, methanol and carbon monoxide poisoning, hepatic encephalopathy, and hypoxic injury can be differentiated on the basis of the clinical history, laboratory findings, and radiologic features. MIE has also well-known features of the reversible symptoms and radiologic findings after the discontinuation of metronidazole administration14) in addition to its typical anatomic involvement of the lesions. Given that all MIE series in previous reports were diagnosed, not by biopsy, but by clinical suspicions, the authors thought that pathologic confirmation would not be necessary in this condition with benign natural history.
In spite of homogenous findings on FLAIR/T2-weighted images, signal intensities of lesions on DWI and ADC map in MIE have been variable. Some of the lesions observed in FLAIR or T2-weighted image appeared as positive in DWI. High-signal intensity lesions on DWI often, but not always, have tended to be of low signal intensity on the ADC map2,14). This variability might have a predictive role in the prognosis of patients with MIE because of the possibility that lesions with high signal in DWI and low signal in ADC map may represent cytotoxic edema, as reported in cerebral infarction14). Complete or near-complete resolution of the original lesions on follow-up MRIs with discontinuation of the drug is one of most striking and typical findings of MIE. However, the lesions are sometimes persistent and it is not clear whether irreversibility of the lesions depends on initial low ADC11) or some anatomical preponderance such as corpus callosum12) or inferior olivary nuclei16). Kim et al.12) proposed that most of the abnormal signal intensities in MIE might correspond to areas of vasogenic edema, except for lesions in the corpus callosum. Lee at al.14) suggested that the lesions with high signal on DWIs and low signal on ADC maps mostly indicate cytotoxic edema rather than vasogenic edema. Therefore, lesions with low ADC value or lesions located in corpus callosum might be regarded as poor prognostic factors in MIE. In our case, all the lesions including that in subcortical white matter, which produce a low signal on the ADC map, disappeared after discontinuation of metronidazole. However, abnormal signal intensity in the splenium of the corpus callosum persisted even after 4 weeks of follow-up. Therefore, irreversibility of the lesions in MIE might depend on specific anatomic location such as splenium of corpus callosum, rather than a low ADC map.
Given that the treatment of MIE is discontinuation of metronidazole, substitutes for metronidazole would be needed when there is a remaining abscess. Presentations of MIE in brain abscess are very rare2,14). Therefore, a management strategy for remaining brain abscess has not been clearly described when metronidazole was withheld due to MIE, especially for those with anaerobic brain abscess. In our case, anaerobic brain abscess did not respond to a single regimen of meropenem chosen as an alternative to metronidazole7) when metronidazole-induced transient neutropenia had occurred. Therefore, we chose a combined regimen of meropenem and levofloxacin for the remaining abscess when MIE had occurred. Meropenem can be an alternative antibiotic choice for anaerobic species7) and it acts synergistically with levofloxacin5). Through the combined use of these regimen, we successfully treated the remaining abscess in the absence of metronidazole. Most brain abscesses are treated by the neurosurgeons. So, neurosurgeons should keep in mind the possibility of this rare complication when dealing with a brain abscess requiring the long-term use of metronidazole.
Long-term use of metronidazole for anaerobic brain abscess can result in atypical MIE asymmetrically involving thalamus, putamen, midbrain, and subcortical white matter, as well as typical symmetric lesions in medulla, pons, dentate nuclei of cerebellum, and splenium of corpus callosum. A combined regimen of meropenem and levofloxacin can be an alternative option for a remaining abscess in a patient with MIE. Caution should be given when long-term use of metronidazole is indicated in patients with brain abscess.
References
1. Ahmed A, Loes DJ, Bressler EL. Reversible magnetic resonance imaging findings in metronidazole-induced encephalopathy. Neurology. 1995; 45:588–589. PMID: 7898724.

2. Bahn Y, Kim E, Park C, Park HC. Metronidazole induced encephalopathy in a patient with brain abscess. J Korean Neurosurg Soc. 2010; 48:301–304. PMID: 21082066.

3. Bonkowsky JL, Sondrup C, Benedict SL. Acute reversible cerebellar lesions associated with metronidazole therapy. Neurology. 2007; 68:180. PMID: 17224569.

4. Cecil KM, Halsted MJ, Schapiro M, Dinopoulos A, Jones BV. Reversible MR imaging and MR spectroscopy abnormalities in association with metronidazole therapy. J Comput Assist Tomogr. 2002; 26:948–951. PMID: 12488741.

5. Cottagnoud P, Cottagnoud M, Acosta F, Flatz L, Kuhn F, Stucki A, et al. Meropenem prevents levofloxacin-induced resistance in penicillin-resistant pneumococci and acts synergistically with levofloxacin in experimental meningitis. Eur J Clin Microbiol Infect Dis. 2003; 22:656–662. PMID: 14557920.

6. De Bleecker JL, Leroy BP, Meire VI. Reversible visual deficit and Corpus callosum lesions due to metronidazole toxicity. Eur Neurol. 2005; 53:93–95. PMID: 15855780.

7. Glupczynski Y, Berhin C, Nizet H. Antimicrobial susceptibility of anaerobic bacteria in Belgium as determined by E-test methodology. Eur J Clin Microbiol Infect Dis. 2009; 28:261–267. PMID: 18797943.

8. Hammami N, Drissi C, Sebai R, Araar M, Maatallah Y, Belghith L, et al. Reversible metronidazole-induced encephalopathy. J Neuroradiol. 2007; 34:133–136. PMID: 17368540.

9. Heaney CJ, Campeau NG, Lindell EP. MR imaging and diffusion-weighted imaging changes in metronidazole (Flagyl)-induced cerebellar toxicity. AJNR Am J Neuroradiol. 2003; 24:1615–1617. PMID: 13679281.
10. Horlen CK, Seifert CF, Malouf CS. Toxic metronidazole-induced MRI changes. Ann Pharmacother. 2000; 34:1273–1275. PMID: 11098341.

11. Kim DW, Park JM, Yoon BW, Baek MJ, Kim JE, Kim S. Metronidazole-induced encephalopathy. J Neurol Sci. 2004; 224:107–111. PMID: 15450780.

12. Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR imaging of metronidazole-induced encephalopathy : lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol. 2007; 28:1652–1658. PMID: 17885234.

13. Lamp KC, Freeman CD, Klutman NE, Lacy MK. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin Pharmacokinet. 1999; 36:353–373. PMID: 10384859.

14. Lee SS, Cha SH, Lee SY, Song CJ. Reversible inferior colliculus lesion in metronidazole-induced encephalopathy : magnetic resonance findings on diffusion-weighted and fluid attenuated inversion recovery imaging. J Comput Assist Tomogr. 2009; 33:305–308. PMID: 19346865.

15. Rao DN, Mason RP. Generation of nitro radical anions of some 5-nitrofurans, 2- and 5-nitroimidazoles by norepinephrine, dopamine, and serotonin. A possible mechanism for neurotoxicity caused by nitroheterocyclic drugs. J Biol Chem. 1987; 262:11731–11736. PMID: 2887562.

16. Seok JI, Yi H, Song YM, Lee WY. Metronidazole-induced encephalopathy and inferior olivary hypertrophy : lesion analysis with diffusion-weighted imaging and apparent diffusion coefficient maps. Arch Neurol. 2003; 60:1796–1800. PMID: 14676060.

17. Woodruff BK, Wijdicks EF, Marshall WF. Reversible metronidazole-induced lesions of the cerebellar dentate nuclei. N Engl J Med. 2002; 346:68–69. PMID: 11778010.

Fig. 1
Initial brain magnetic resonance image showing a ring-enhanced brain abscess in the left frontal lobe.
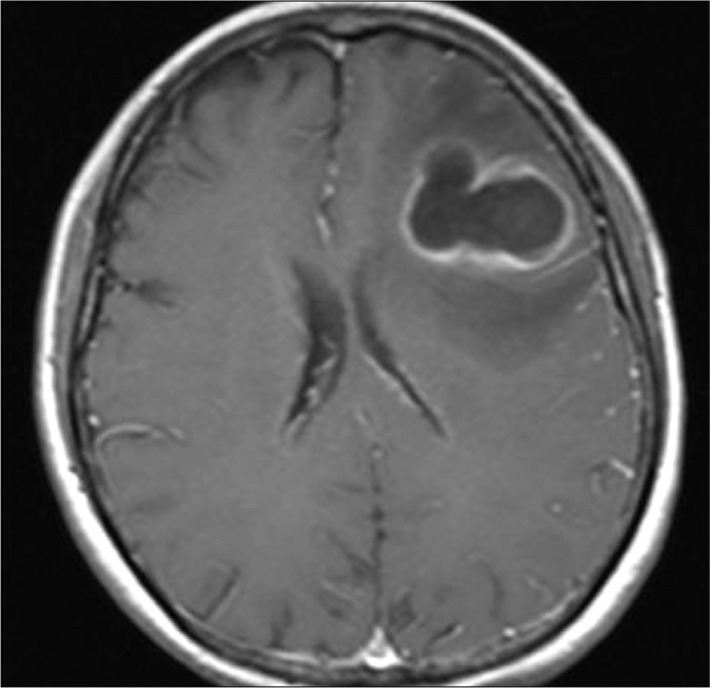
Fig. 2
Fluid attenuated inversion recovery magnetic resonance images after 84 days of metronidazole administration. Symmetric round shaped high-signal intensity at the dorsal medullae, the dentate nuclei (A), and the pons (B) and symmetric involvement along the splenium (E) are apparent. Note multiple asymmetric lesions in midbrain (C), thalamus (D), putamen (E) and subcortical white matter on right side of the brain (F). Diffuse signal change due to previous brain abscess is observed on left frontal area.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download