Abstract
The experience of pediatric deep brain stimulation (DBS) of the globus pallidus internus (GPi) in the treatment of early-onset DYT1 generalized dystonia is still limited. Here, we report the surgical experience of bilateral GPi-DBS under general anesthesia by using microelectrode recording in a 7-year-old girl with early-onset DYT1 generalized dystonia. Excellent improvement of her dystonia without neurological complications was achieved. This case report demonstrates that GPi-DBS is an effective and safe method for the treatment of medically refractory early-onset DYT1 generalized dystonia in children.
The most common cause of early-onset idiopathic generalized dystonia, previously known as primary idiopathic generalized dystonia, is DYT1 dystonia, caused by a 3 base pair deletion in the TOR1A gene2). DYT1 dystonia typically presents in childhood and usually starts in a limb, gradually progressing to a generalized form. This condition can cause significant disability. Deep brain stimulation (DBS) has been reported to be aid in the management of chronic drug refractory primary dystonia for individuals 7 years of age or older9). DBS at the globus pallidus internus (GPi) has been established as a safe and effective modality for controling medically refractory idiopathic generalized dystonia4,11). However, the experience of pediatric GPi-DBS for the treatment of early-onset DYT1 generalized dystonia is still limited. A case of pediatric DBS for DYT1 dystonia has not been published in Korea. Here, we report the surgical experience of bilateral GPi-DBS in a 7-year-old girl with early-onset DYT1 generalized dystonia.
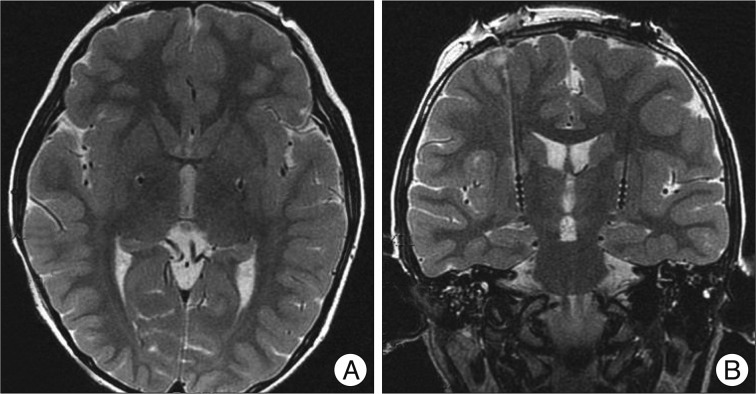
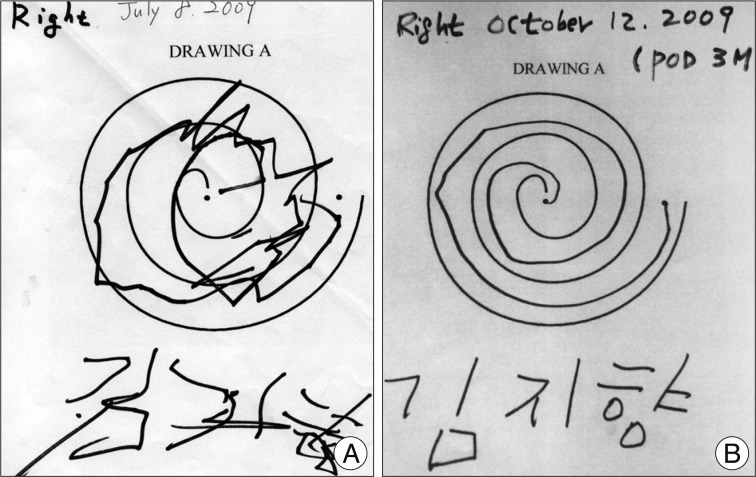
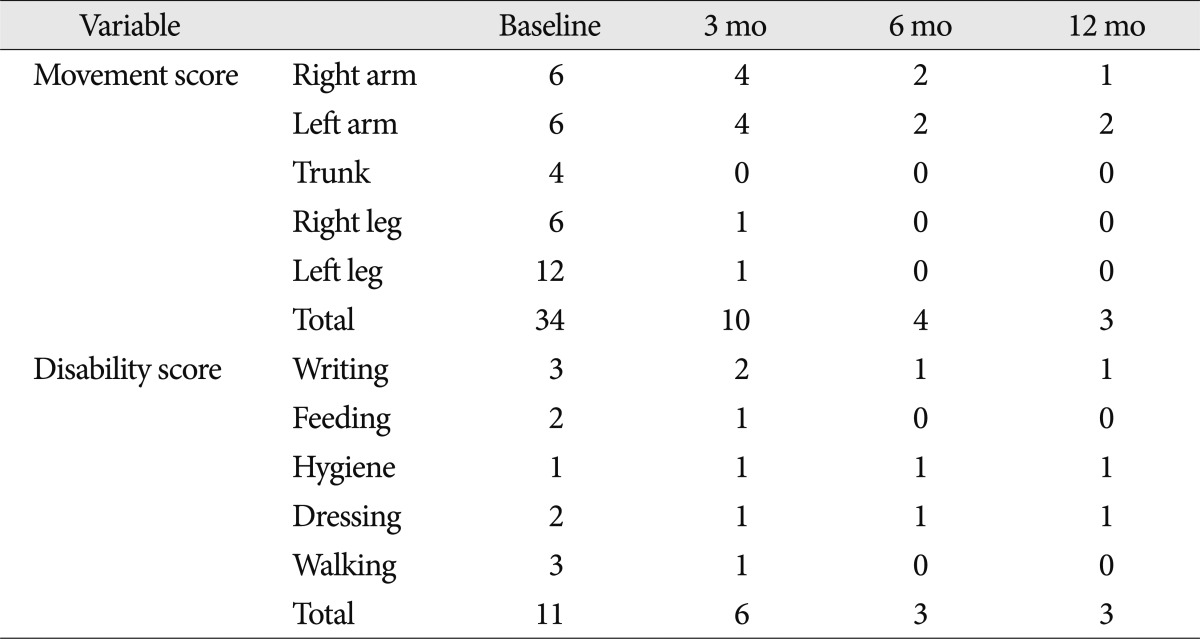
A 7-year-old girl with genetically confirmed DYT1 dystonia was referred for possible surgical treatment of medically intractable progressive generalized dystonia. She was relatively healthy until October 2007. Her dystonia started in the right arm, accompanied by difficulty in writing. The dystonia then progressed, first involving her left upper limb and then, the lower legs and finally her hip and trunk in April 2009. She had difficulty in writing, dystonic flexion of the left knee, and severely impaired walking or requiring assistance, with difficulty in maintaining an erect standing position. Moreover, she had difficulty in feeding herself. Brain magnetic resonance imaging (MRI) and medical laboratory evaluation did not show any abnormal findings. Genetic testing for DYT1 was performed in April 2008, which gave a positive result for DYT1 gene. Her father and one uncle were also positive for DYT1, whereas her mother was negative for this mutation. Her father experienced writing tremor in the right hand, and her uncle had mild dystonia in the neck and the right hand. She showed little response to a various medications, including anticholinergic agents, such as rivotril, levetiracetam, and tetrabenazine. Moreover, these medications had side effects such as lethargy and hallucination. After the failure of all available medications for dystonia, she underwent simultaneous bilateral implantation of quadripolar DBS leads (DBS Model 3387, Medtronic) into the GPi under sevoflurane inhalation general anesthesia on July 9, 2009. The electrode target was based on indirect and direct targeting with a 1.5-tesla MRI (General Electric, USA) and Neurosurgery Simulator® (Dimos, Seoul, Korea) as surgical planning tools for refined microelectrode recording (MER). The mean distance between the anterior and posterior commissure was 22.5 mm. The arcs and sliding angles were 65° and 5° on the left and 70° and 7° on the right, respectively. MER was performed using 2 channels at the central and medial sides. The implanted central target was 2.8 mm anterior to the mid-commissural point, 21.5 mm lateral to the midline, and 4 mm below the anterior-posterior commissural plane (Fig. 1). The length of the GPi in the central final target was measured at 7 mm in the left and 6 mm in the right. Intraoperative test stimulation with a microelectrode was performed at 1.0-4.0 V, 200 µs, and 130 Hz to check motor response. The electrode was fixed on the cranium by using a titanium microplate instead of a burr hole ring and cap. After 3 days of a trial test (Model 3625 external tester, Medtronic Inc.) in the hospital ward, the stimulation device (Soletra, Medtronic Inc.) was implanted subcutaneously in the subclavicular area and a trough was drilled in the posterior parietal bone to secure the connector. The stimulation parameters of the test stimulation in bipolar mode were as follows : 0-2+; frequency, 130 Hz; pulse width, 60 µs; amplitude, 3.0 V. During the test stimulation, the patient showed moderate improvement, she described her satisfaction and reported that she was able to sleep comfortably without sustained muscle contraction and abnormal flexed posture in the left knee that were present before the surgery. No significant peri-operative complication occurred. Progressive and sustained improvement of her dystonia was noted. She was able to walk with an erect posture at 10 days and run at 3 months after the DBS surgery (Fig. 2). Her dystonia disappeared almost completely, except for some difficulty in hand-writing (Fig. 3). The Burke-Fahn-Martin Dystonia Rating Scale (BFMDRS) total movement scores were 34/120 in the preoperative period, 10/120 at 3 months after the surgery, 4/120 at 6 months after the surgery and 3/120 at 12 and 30 months after the surgery. The BFMDRS total disability scores were 11/30 in the preoperative period, 6/30 at 3 months after the surgery, 3/30 at 6 months after the surgery and 3/30 at 12 and 30 months after the surgery (Table 1). The stimulation parameters in monopolar mode were as follows : 0-case+; frequency, 130 Hz; pulse width, 120 µs; amplitude, 2.8 V on the right side, and 3.3 V on the left side at the last follow-up. No significant complications were encountered.
We experienced the efficacy and safety of bilateral GPi-DBS in a 7-year-old girl with early-onset DYT1 generalized dystonia with a follow-up period of 30 months. Her prognosis showed a 70.6% improvement in the BFMDRS total movement scores at 3 months, 88.2% improvement at 6 months, and 91.2% improvement at 1 year, then the clinical benefit was maintained up to the last follow-up. These results correspond well with those of a number of recent series reporting that bilateral GPi-DBS is a highly effective treatment for early-onset idiopathic generalized dystonia. Coubes et al.4) reported a mean 79% improvement in the BFMDRS motor subscore and a mean 65% improvement in the disability subscore 2 years after the surgery in 31 patients with primary generalized dystonia. Isaias et al.7) reported a short disease duration (<15 years) at the time of surgery predicted a superior response to DBS. The phasic symptoms improved more rapidly than fixed postures, the presence of which may limit functional recovery8,12). When prolonged dystonia results in fixed contractures, additional orthopedic surgery may be required to maximize functional gains1). Early DBS surgery for primary generalized dystonia has been proposed after drug treatment has failed and before fixed skeletal deformities have developed. Pediatric DBS presents several challenges with respect to surgical planning and technique, such as identification of anatomical landmarks, performing awake surgery or placing the patient under general anesthesia, and hardware-related problems6,10). More than 90% of brain development is believed to be completed by the age of 7 years5). Thus, it seems reasonable to use nearly similar landmarks in children aged ≥7 years as those used in adults. Performing awake surgery in children is extremely difficult in general, but it may be impossible in these cases because of the dystonic state or cooperation problems during the DBS procedure10). In our patient, the use of general anesthesia was more feasible because she was too young to be expected to cooperate during the procedure. The need for MER in pallidal DBS is debated; however, MER provides important information for refining anatomical targeting under general anesthesia. Multichannel MER particularly influences the final electrode placement to precisely identify the target structure and its borders. Microelectrode mapping was possible, even under general anesthesia as sevoflurane was used our case6). Moreover, testing for thresholds of side effects in the form of capsular effects can be performed sufficiently under general anesthesia10). In our case, intraoperative internal capsular response with microelectrode stimulation was not noted within the range of 1.0-4.0 V. Postoperative infection related to the DBS device has been reported to be higher in children than in adults, with incidence rates of 5-33%3). We anchored the DBS electrode by using a microplate, and drilled a trough in the cranial surface to secure the connector to avoid possible infection and cosmetic problems.
References
1. Alterman RL, Tagliati M. Deep brain stimulation for torsion dystonia in children. Childs Nerv Syst. 2007; 23:1033–1040. PMID: 17551738.

2. Augood SJ, Penney JB Jr, Friberg IK, Breakefield XO, Young AB, Ozelius LJ, et al. Expression of the early-onset torsion dystonia gene (DYT1) in human brain. Ann Neurol. 1998; 43:669–673. PMID: 9585364.

3. Constantoyannis C, Berk C, Honey CR, Mendez I, Brownstone RM. Reducing hardware-related complications of deep brain stimulation. Can J Neurol Sci. 2005; 32:194–200. PMID: 16018154.

4. Coubes P, Cif L, El Fertit H, Hemm S, Vayssiere N, Serrat S, et al. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia : long-term results. J Neurosurg. 2004; 101:189–194. PMID: 15309907.

5. Dekaban AS. Changes in brain weights during the span of human life : relation of brain weights to body heights and body weights. Ann Neurol. 1978; 4:345–356. PMID: 727739.

6. Gross RE, Mewes K, Ghani G. Bakay RA, editor. Anesthesia for movement disorder surgery. Movement Disorder Surgery. The Essentials. 2008. New York: Stuttgart;p. 70–82.
7. Isaias IU, Alterman RL, Tagliati M. Outcome predictors of pallidal stimulation in patients with primary dystonia : the role of disease duration. Brain. 2008; 131:1895–1902. PMID: 18567622.

8. Jeong SG, Lee MK, Kang JY, Jun SM, Lee WH, Ghang CG. Pallidal deep brain stimulation in primary cervical dystonia with phasic type : clinical outcome and postoperative course. J Korean Neurosurg Soc. 2009; 46:346–350. PMID: 19893724.

9. Marks WA, Honeycutt J, Acosta F, Reed M. Deep brain stimulation for pediatric movement disorders. Semin Pediatr Neurol. 2009; 16:90–98. PMID: 19501337.

10. Pinsker MO, Volkmann J, Falk D, Herzog J, Steigerwald F, Deuschl G, et al. Deep brain stimulation of the internal globus pallidus in dystonia : target localisation under general anaesthesia. Acta Neurochir (Wien). 2009; 151:751–758. PMID: 19468677.

11. Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia : a prospective 3 year follow-up study. Lancet Neurol. 2007; 6:223–229. PMID: 17303528.

12. Zorzi G, Marras C, Nardocci N, Franzini A, Chiapparini L, Maccagnano E, et al. Stimulation of the globus pallidus internus for childhood-onset dystonia. Mov Disord. 2005; 20:1194–1200. PMID: 15895426.

Fig. 1
Postoperative MRI axial T2-weighted (A) and coronal T2-weighted (B) images showing the electrodes within the posteroventral globus pallidus internus.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download