Abstract
Intracranial extraskeletal myxoid chondrosarcoma is extremely rare, with only seven patients previously reported. We present a case report of a 21-year-old woman admitted for weakness in her right extremities and symptoms of increased intracranial pressure. Magnetic resonance imaging (MRI) revealed hydrocephalus and a well-enhanced large mass around her left thalamus. A left parietal craniotomy and a cortisectomy at the superior parietal lobule were performed. Total surgical resection was also performed, and pathology results confirmed an extraskeletal myxoid chondrosarcoma. Postoperative MRI showed no residual tumor, and the patient underwent radiotherapy. After six months of radiotherapy, the patient's headache and weakness had improved to grade IV. This malignant tumor showed high rates of recurrence in previous reports. We here report another occurrence of this highly malignant and rare tumor in a patient treated using total surgical excision and adjuvant radiotherapy.
Intracranial myxoid chondrosarcoma is extremely rare and is thought to arise from the choroid plexus, dura, or in rare instances the pineal region1,3,6-8,12,17). Since its first description in 1972, only seven intracranial extraskeletal myxoid chondrosarcoma tumors have been reported2,4,5,8,13-15,17). We describe a patient with an intracranial myxoid chondrosarcoma originating from the choroid plexus of the left lateral ventricle.
A 21-year-old woman was admitted with weakness in the right extremities that had been persisted for over a month. She had a known brain tumor, which has been diagnosed in another country. At that time, she experienced headaches as well as visual and hearing disturbances. Previous brain magnetic resonance imaging (MRI) showed a well-enhanced mass, measuring 26×40×29 mm, in the posterior horn of the left lateral ventricle. Ventricular dilatation was absent, and peritumoral edema was observed. For financial reasons, however, she was not treated at that time.
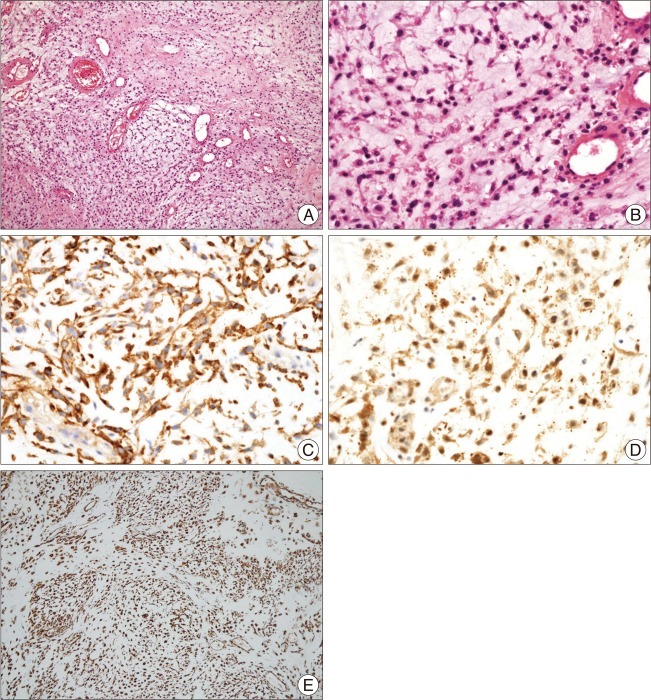
Upon admission to our hospital, she complained of both visual and hearing loss. She also showed a grade II weakness of the right extremities. MRI revealed hydrocephalus and a 32×63×48-mm sized well-enhanced mass around the right thalamus. We also observed severe compression of the third ventricle and diffuse peritumoral edema. An enhanced MRI scan revealed a lobulated, heterogeneous enhanced tumor (Fig. 1). A computed tomography (CT) scan of her brain also showed a well-enhanced mass, as well as multiple lobulated spots with hypodensity within the mass. To decompress her increased intracranial pressure, we underwent operation to remove the mass completely, using a transcortical approach to the posterior horn of the left lateral ventricle. A left parietal craniotomy and a cortisectomy at the superior parietal lobule were performed. The tumor was brown in color, and the margin between the brain parenchyma and supporting tissue was clearly distinguishable. The tumor was hypervascularized, hard, and lobulated, and it was completely removed in piecemeal fashion. A frozen biopsy of the tumor suggested that it was a pilocytic astrocytoma. The tumor was found to be composed of strands or cords of oval and spindle cells embedded in abundant myxoid stroma (Fig. 2A). The tumor cells had relatively uniform oval nuclei with dense, evenly dispersed chromatin, and a moderate amount of eosinophilic cytoplasm that was often finely vacuolated (Fig. 2B). Mitotic figures were rarely observed.
The tumor cells were further examined by immunohistochemistry, and antibodies were used at the dilutions listed. Tumor cells were found to be focally and strongly positive for epithelial membrane antigen (1 : 25, Dako, Glostrup, Denmark) (Fig. 2C), weakly positive for class III β-tubulin (1 : 200, clone TU-20, Genetex, Irvine, CA, USA), diffusely positive for microtubule-associated protein 2 (1 : 200, clone AP18, Neomarkers, Fremont, CA, USA) (Fig. 2D), and positive for vimentin (1 : 250, Zymed, San Francisco, CA, USA) (Fig. 2E). In contrast, the tumor cells were negative for S-100 protein (1 : 1000, Zymed, San Francisco, CA, USA), cytokeratin (1 : 250, Zymed San Francisco, CA, USA), and glial fibrillary acidic protein (GFAP, 1 : 200, Biogenex, San Ramon, CA, USA). Final pathologic analysis of the above results led to a diagnosis of extraskeletal myxoid chondrosarcoma. Postoperative MRI showed no residual tumor. The patient then underwent adjuvant radiotherapy, at a total dose of 6080 cGy, as well as rehabilitation. After six months of treatment, the headache and weakness symptoms had improved to grade IV, but other neurologic deficits, including blindness and deafness, were unchanged.
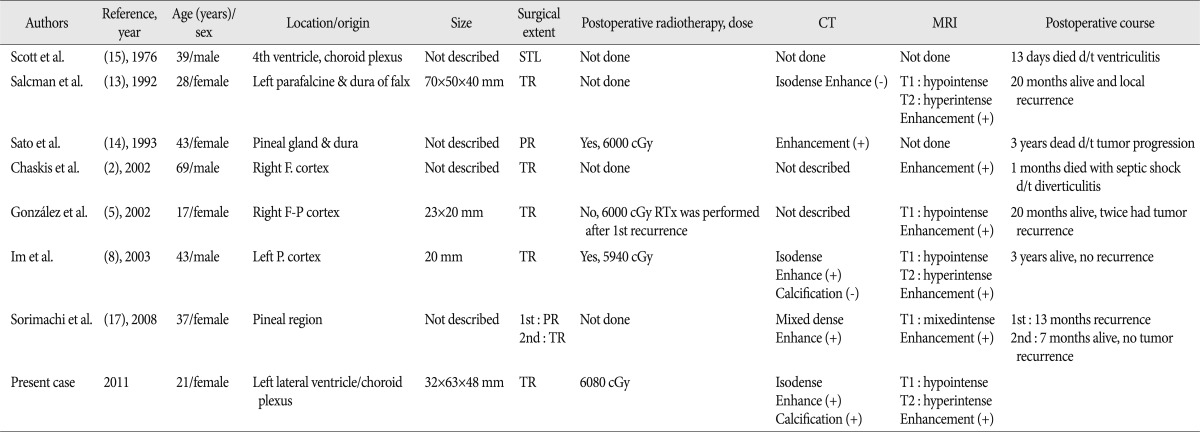
Histologically, three subtypes of cranial and intracranial chondrosarcomas have been described : classic, mesenchymal, and myxoid5,7,9). Intracranial extraskeletal myxoid chondrosarcomas are extremely rare, with only seven cases previously reported to date2,5,8,13-15,17). A summary of these previous patients, including imaging results, the surgical extent of the tumor, postoperative radiation treatment, and patient outcomes is shown in Table 1.
Intracranial extraskeletal myxoid chondrosarcomas are thought to originate from the dura, leptomeninges, parenchyma, and choroid plexus2,5,8,13-16). Our findings in the present case suggest that the tumor originated from the choroid plexus of the lateral ventricle.
Preoperative imaging methods, including CT and MRI, revealed similar results in previous patients with intracranial extraskeletal myxoid chondrosarcomas. Precontrast CT scans have shown isodensity of tumors in five of the seven previously reported patients; however, it should be noted that the two remaining patients had preoperative tumor bleeding16,17). Most previous tumors of this type were calcified, with one showing greater than 50% calcification10). All of these previous tumors also showed contrast enhancement on CT scans. T1-weighted MRI scans usually showed hypointensity of these tumors, whereas T2-weighted MRI scans usually showed hyperintensity. Gadolinium-enhanced MRI typically showed enhancement of the tumors13). Previous immunohistochemical analyses of intracranial myxoid chondrosarcomas have shown that all seven of the previously reported cases expressed vimentin, with some expressing epithelial membrane antigen, neurofilament, and synaptophysin. In contrast, S-100 staining is very rare and usually focal10,11).
The optimal treatment for intracranial myxoid chondrosarcoma is radical excision, and total removal of the tumor is critical5,7,10,12,13). In six of the eight patients diagnosed with this tumor to date, including our current patient, the tumors were completely removed by surgical excision2,5,8,13,17). Fortunately, these tumors had good dissection margins from the surrounding tissue, and tumor adhesions were not severe13). However, it is difficult to completely remove tumors located in the cerebellopontine angle, the pineal region, or the sellar portion of the brain. In addition, intracranial myxoid chondrosarcomas are not associated with a good prognosis after treatment. Of the seven patients previously described, two showed tumor recurrences after total resection5,10,12,13). Of the remaining five patients, two showed tumor recurrences and two died due to postoperative complications2,14,15,17).
Adjuvant therapies, including radiotherapy, brachytherapy, and proton beam treatment, have been found to improve patient outcomes for this rare cancer5,9,10,13). Postoperative radiotherapy was administered to one of the three earlier patients who underwent partial tumor removal14,15,17) and to two of the five patients who underwent total removal, with radiation doses ranging from 5940 to 6000 cGy2,5,8,13,17). Following removal of the tumor, our patient received 6080 cGy adjuvant radiation therapy. To date, there have been no reports on the advantages of adjuvant chemotherapy for patients with intracranial extraskeletal myxoid chondrosarcomas.
We describe a case of intracranial extraskeletal myxoid chondrosarcoma and review the literature on these rare tumors. Despite their malignant nature, these tumors have well-defined margins and are clearly distinct from normal brain tissue. Intracranial extraskeletal myxoid chondrosarcoma has a high rate of recurrence in previous reports. Here, we present a case report of this tumor treated with total tumor resection and adjuvant radiotherapy.
References
1. Bourgouin PM, Tampieri D, Robitaille Y, Robert F, Bergeron D, del Carpio R, et al. Low-Grade Myxoid Chondrosarcoma of the Base of the Skull: CT, MR, and Histopathology. J Comput Assist Tomogr. 1992; 16:268–273. PMID: 1545025.
2. Chaskis C, Michotte A, Goossens A, Stadnik T, Koerts G, D'Haens J. Primary intracerebral myxoid chondrosarcoma. Case illustration. J Neurosurg. 2002; 97:228. PMID: 12134922.
3. Cummings TJ, Bridge JA, Fukushima T. Extraskeletal myxoid chondrosarcoma of the jugular foramen. Clin Neuropathol. 2004; 23:232–237. PMID: 15581026.
4. Enzinger FM, Shiraki M. Extraskeletal myxoid chondrosarcoma. An analysis of 34 cases. Hum Pathol. 1972; 3:421–435. PMID: 4261659.
5. González-Lois C, Cuevas C, Abdullah O, Ricoy JR. Intracranial extraskeletal myxoid chondrosarcoma : case report and review of the literature. Acta Neurochir (Wien). 2002; 144:735–740. PMID: 12181708.
6. Grossman RI, Davis KR. Cranial computed tomographic appearance of chondrosarcoma of the base of the skull. Radiology. 1981; 141:403–408. PMID: 7291563.

7. Hassounah M, Al-Mefty O, Akhtar M, Jinkins JR, Fox JL. Primary cranial and intracranial chondrosarcoma. A survey. Acta Neurochir (Wien). 1985; 78:123–132. PMID: 3911744.
8. Im SH, Kim DG, Park IA, Chi JG. Primary intracranial myxoid chondrosarcoma : report of a case and review of the literature. J Korean Med Sci. 2003; 18:301–307. PMID: 12692436.

9. Korten AG, ter Berg HJ, Spincemaille GH, van der Laan RT, Van de Wel AM. Intracranial chondrosarcoma : review of the literature and report of 15 cases. J Neurol Neurosurg Psychiatry. 1998; 65:88–92. PMID: 9667567.

10. O'Brien J, Thornton J, Cawley D, Farrell M, Keohane K, Kaar G, et al. Extraskeletal myxoid chondrosarcoma of the cerebellopontine angle presenting during pregnancy. Br J Neurosurg. 2008; 22:429–432. PMID: 18568733.
11. Oliveira AM, Sebo TJ, McGrory JE, Gaffey TA, Rock MG, Nascimento AG. Extraskeletal myxoid chondrosarcoma : a clinicopathologic, immunohistochemical, and ploidy analysis of 23 cases. Mod Pathol. 2000; 13:900–908. PMID: 10955458.

12. Sala F, Talacchi A, Beltramello A, Iuzzolino P, Bricolo A. Intracranial myxoid chondrosarcoma with early intradural growth. J Neurosurg Sci. 1998; 42:159–163. PMID: 10192057.
13. Salcman M, Scholtz H, Kristt D, Numaguchi Y. Extraskeletal myxoid chondrosarcoma of the falx. Neurosurgery. 1992; 31:344–348. PMID: 1513440.

14. Sato K, Kubota T, Yoshida K, Murata H. Intracranial extraskeletal myxoid chondrosarcoma with special reference to lamellar inclusions in the rough endoplasmic reticulum. Acta Neuropathol. 1993; 86:525–528. PMID: 8310804.

15. Scott RM, Dickersin R, Wolpert SM, Twitchell T. Myxochondrosarcoma of the fourth ventricle. Case report. J Neurosurg. 1976; 44:386–389. PMID: 1249620.
16. Smith TW, Davidson RI. Primary meningeal myxochondrosarcoma presenting as a cerebellar mass : case report. Neurosurgery. 1981; 8:577–581. PMID: 7266799.
17. Sorimachi T, Sasaki O, Nakazato S, Koike T, Shibuya H. Myxoid chondrosarcoma in the pineal region. J Neurosurg. 2008; 109:904–907. PMID: 18976082.

Fig. 1
Imaging findings for the current patient. A : T1-weighted magnetic resonance imaging (MRI) showing homogeneous iso-signal intensity of a 63-mm tumor in the left lateral ventricle along with ventricular dilatation. B : T2-weighted MRI showing a heterogeneous high signal intensity tumor and peritumoral edema. C : T1-weighted enhanced MRI showing strong enhancement of the tumor.
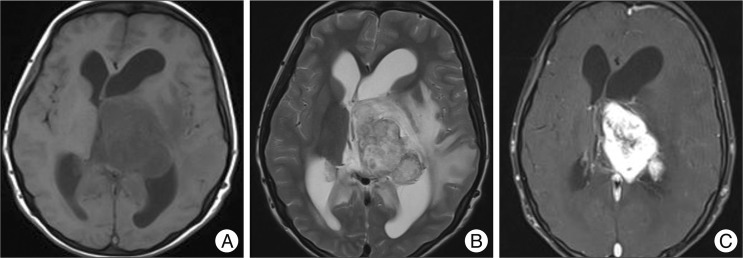
Fig. 2
Histologic features of the tumor in the current patient. A : The tumor consists of strands or cords of oval cells and abundant myxoid stroma (H&E, ×100). B : The tumor cells interconnected to form cords and had relatively uniform oval nuclei and a moderate amount of eosinophilic cytoplasm (H&E, ×400). C, D and E : These tumor cells are positive for epithelial membrane antigen (original magnification ×400) (C), microtubule-associated protein 2 (original magnification ×400) (D), and vimentin (original magnification ×100) (E) by immunohistochemical staining.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download