Abstract
Intraventricular cavernous hemangiomas are uncommon. Among them, those occurred at the foramen of Monro in the third ventricle may be of particular interest because of its rarity, development of hydrocephalus, being differentiated from other brain lesions. We present a rare case of intraventricular cavernous hemangioma at foramen of Monro which was resected through microsurgery and also review the relevant literatures.
Cavernous hemangioma constitutes 5-10% of vascular malformations occurring in the entire central nervous system (CNS). Intraventricular location constituting 2.5-10.8% of cerebral cavernous hemangioma is uncommon because most of them are usually found in the parenchyme of CNS4). So far, the intraventricular cavernous hemangioma has been reported to be less than 90 cases to our best knowledge. Those located in the third ventricle account for approximately half of all intraventricular cavernous hemangiomas. In particular, cavernoma at the foramen of Monro which have been reported to be about 13 cases are very rare and may be difficult to differentiate from other brain tumors1,4). Herein, we present an additional case of cavernous hemangioma located in the foramen of Monro of the third ventricle and review the relevant literatures.
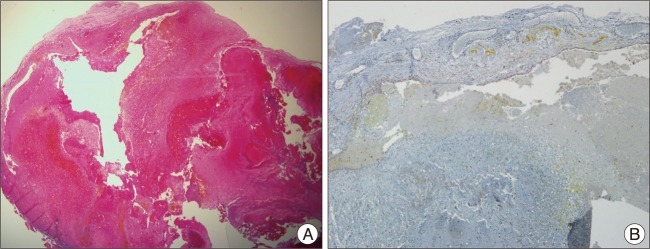
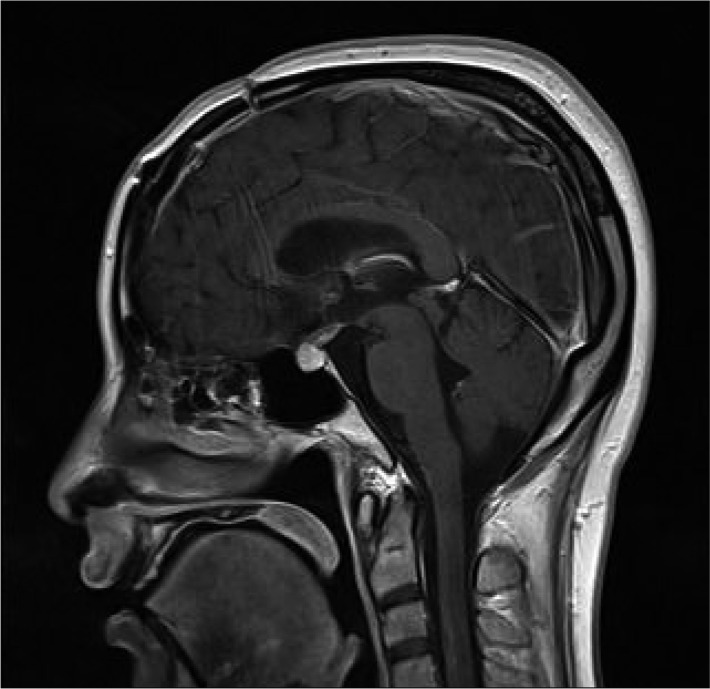
A 30-year-old female patient who was pregnant presented with intermittent non-throbbing headache for a month before admission. She has often suffered from short-term memory deficits gradually. She complained the projectile vomiting and fever without consciousness disturbance which were observed shortly after normal vaginal delivery. She was referred to department of neurology under the impression of meningitis. On physical examinations, definite neurological deficits were not found. Computed tomogram (CT) showed enlargement of both lateral ventricles which may be caused by 3 cm sized multi-lobular calcified mass with slightly peripheral rim enhancement at the foramen of Monro in the third ventricle. The lesion seemed to involve the left thalamus parenchyme partially. Magnetic resonance image (MRI) revealed a relatively well-delineated mass at the foramen of Monro with heterogeneous signal intensity on both T1 and T2 weighted images. The surrounding rim of low signal intensity around mass was not delineated definitely on T2-weighted image. A peri-lesional edema in the left thalamus was observed on the FLAIR image and only minimal peripheral rim enhancement around mass was found on enhanced T1-weighted image (Fig. 1). Conventional cerebral angiograms revealed no vascular abnormalities. Anterior interhemispheric trans-callosal approach was performed to remove the intraventricular lesion. Operative findings showed that the mass with xanthochromic and lobular surface obstructing the foramen of Monro was located in the third ventricle. The left posterior margin of the mass was close contact to the thalamus while resection. The mass was removed totally without significant bleeding (Fig. 2). The histological examination revealed large hyalinized vessels filled with organizing thrombus. Immunostaining for smooth muscle actin (SMA) was positive in the vessel wall (Fig. 3). Those findings were compatible with characteristics of cavernous hemangioma. After operation, the patient's symptoms including vomiting and headache were recovered gradually (Fig. 4).
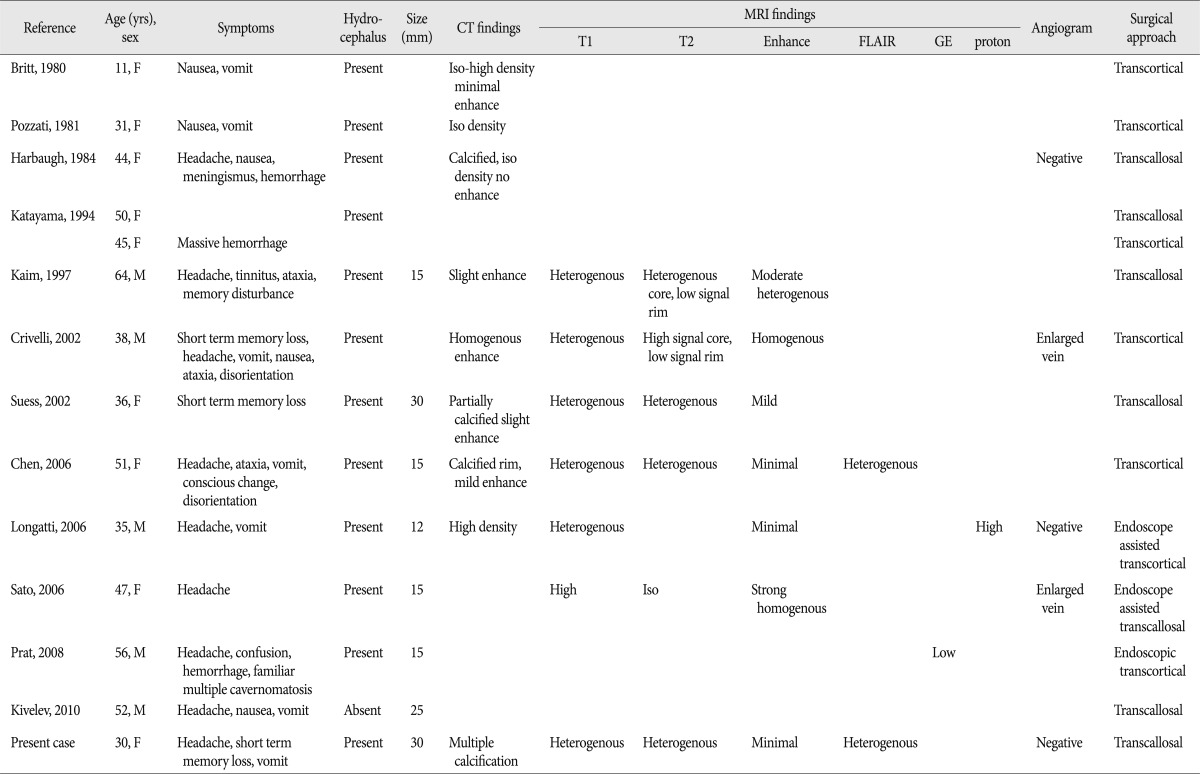
Cavernous hemangiomas known as cavernous malformations, cavernous angiomas or cavernomas are a kind of vascular malformation which is angiographically occult unlike arteriovenous malformations. These benign lesions are composed of sinusoidal vascular channels which are lined with a single layer endothelium predisposing to hemorrhage and have perilesional surrounding gliosis2). Hemorrhages at different stages due to repeated bleeding, occlusion and thrombosis in the vascular channels are found within the lesion. Organization of the hematoma results in chronic granulation and scar formation. Progressive pseudotumorous evolutions are induced by repeated bleeding which occur in the immediate vicinity of cavernous hemangioma, leading to hemosiderin deposits and gliosis2). Most of them occur in the intra-axial and supratentorial regions and they are typically located in the subcortical deep white matters, basal ganglia, brain stem and cerebellum. However, they have rarely seen in the intraventricular or extra-axial locations4). Kivelev et al.4) summarized intraventricular cavernomas of 89 cases including their own cases and reported that 34 cases (38%) of them were located in the third ventricle. Third ventricular cavernomas can be classified into suprachiasmatic, foramen of Monro, lateral wall, and floor regions depending on the location3). Those located at the foramen of Monro may have particular clinical interests because of hydrocephalus due to foraminal obstruction and have been reported to be only 13 cases so far1,4,5). It is notable that our case can be additional one as foramen of Monro cavernomas of third ventricle. Clinical symptoms of intraventricular cavernomas may be elicited by mass and increased intracranial hypertension due to acute bleeding or obstruction of cerebrospinal fluid pathway. In our case, headache and vomiting resulted from increased intracranial pressure due to obstructive hydrocephalus induced by cavernoma located at the foramen of Monro. Fourteen intraventricular cavernomas located at the foramen of Monro including our case are summarized in the Table 1. There were 5 males and 9 females whose age distribution is ranged from 11 years to 64 years. The clinical findings were symptoms associated with hydrocephalus in most cases, hemorrhages in 3 cases, focal neurological deficits in 5 cases. In all patients, surgical removal was performed completely without significant neurological deficits using the microsurgery or/and endoscopic surgery through the trans-callosal or trans-cortical approaches. Postoperative shunt surgery was not required in most cases. Most of them often showed calcification on computed tomogram (CT) and heterogenous signal intensities and mild enhancement on magnetic resonance image (MRI). Typical CT findings of cavernous hemangiomas in the third ventricle show moderately hyper-dense lobular lesions with mild contrast enhancement. Unlike arteriovenous malformations, they are angiographically occult because cavernous hemangiomas usually do not have feeding or draining vessels obviously. Therefore, MRI has been useful in diagnosis of clinically silent and angiographically occult cavernomas. Common MRI features include a hyper to iso-intense center correlated to the hemorrhages at different stage on T1-weighted images and a surrounding hemosiderin rim of low intensity caused by calcification or fibrosis on T2-weighted images. Perilesional edema is unusual and contrast enhancement is various. And also, it has been reported that typical perilesional hemosiderotic rim might be thinner or absent and core of lesion could be less heterogenous in the intraventricular cavernomas compared with intraparenchymal cavernomas4). Our case showing heterogenous signal intensities in the center of lesion and unclear hemosiderin rim might be confused with other brain tumor lesions. It is considered that additional gradient echo sequences which can detect the hemorrhages in different stages can help differentiate from hemorrhagic form of cavernous hemangioma. Anaplastic astrocytomas, glioblastomas and oligodendrogliomas showing heterogeneous signals on MRI may mimic cavernous hemangiomas because of intratumoral necrosis, hemorrhage or calcification. However, tumor is usually non-hemorrhagic and surrounded by prominent perilesional edema. Cystic and hemorrhagic metastases, which may occur with melanoma, adenocarcinoma has multiple lesions. Furthermore, the third ventricle is a very uncommon site for metastasis or other primary brain tumors. Colloid cysts and germinoma may occur at this location but can be excluded by their different appearance on MRI. Compared with usual radiological findings of cavernomas, our case was not considered as typical cavernoma with peripheral hemosiderin rim and heterogenous internal signal intensities despite normal cerebral angiogram initially. Therefore, vascular lesions such as arteriovenous malformation, venous angioma, tumorous lesions such as glioma, central neurocytoma, subependymal giant cell astrocytoma, colloid cyst, craniopharyngioma, metastasis, meningioma, teratoma, germinoma should be differentiated through full sequences of MRI, angiogram, biopsy and microsurgery4). In our case, this lesion was initially interpretated as tumor such as central neurocytoma, but confirmed as cavernoma through the microsurgery. With regard to the management of cavernous hemangiomas, only a few reports suggested the effectiveness of radiotherapy. However, proliferation and recurrence have been described in several cases treated conservatively or with radiotherapy alone. Therefore, surgical treatment is considered curative if they are readily accessible although management in asymptomatic patients remains controversial.
References
1. Chen CL, Leu CH, Jan YJ, Shen CC. Intraventricular cavernous hemangioma at the foramen of Monro : case report and literature review. Clin Neurol Neurosurg. 2006; 108:604–609. PMID: 15916846.

2. Clatterbuck RE, Eberhart CG, Crain BJ, Rigamonti D. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry. 2001; 71:188–192. PMID: 11459890.

3. Katayama Y, Tsubokawa T, Maeda T, Yamamoto T. Surgical management of cavernous malformations of the third ventricle. J Neurosurg. 1994; 80:64–72. PMID: 8271024.

4. Kivelev J, Niemelä M, Kivisaari R, Hernesniemi J. Intraventricular cerebral cavernomas : a series of 12 patients and review of the literature. J Neurosurg. 2010; 112:140–149. PMID: 19408982.

5. Prat R, Galeano I. Endoscopic resection of cavernoma of foramen of Monro in a patient with familial multiple cavernomatosis. Clin Neurol Neurosurg. 2008; 110:834–837. PMID: 18584950.

Fig. 1
Computed tomogram (CT) shows enlargement of both lateral ventricles which is caused by 3 cm sized multi-lobular calcified mass with slightly peripheral rim enhancement. It is located at the foramen of Monro in the third ventricle and seems to be close to the left thalamus parenchyme partially (A). Magnetic resonance image (MRI) reveals a relatively well-delineated third ventricular mass with heterogeneous signal intensities in the center of lesion on T1-weighted image (B). Typical peripheral hemosiderin rim of low signal intensity is not delineated on T2-weighted image (C). Minimal peripheral enhancement at the right posterior portion of the mass is seen on enhanced T1-weighted image (D). There is a minimal perilesional edema in the left thalamus on the FLAIR image (E). The mass around foramen of Monro is seen on enhanced sagittal T1-weighted image (F).

Fig. 2
Operative finding shows that the mass with xanthochromic and lobular surface obstructing the foramen of Monro is located in the third ventricle. The left posterior margin of the mass is close to the thalamus.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download