Abstract
Objective
The purpose of this study was to evaluate the different patterns of bone loss between the lumbar spine and the femur after ovariectomy in rats.
Methods
Twenty-four female Sprague-Dawley rats underwent a sham operation (the sham group) or bilateral ovariectomy (the ovariectomized group). Four and eight weeks after operation, six rats from each of the two groups were euthanized. Serum biochemical markers of bone turnover including osteocalcin and alkaline phosphatase (ALP), which are sensitive biochemical markers of bone formation, and the telopeptide fragment of type I collagen C-terminus (CTX), which is a sensitive biochemical marker of bone resorption, were analyzed. Bone histomorphometric parameters of the 4th lumbar vertebrae and femur were determined by micro-computed tomography.
Results
Ovariectomized rats were found to have higher osteocalcin, ALP and CTX levels than sham controls. Additionally, 8 weeks after ovariectomy in the OVX group, serum levels of osteocalcin, ALP and CTX were significantly higher than those of 4 weeks after ovariectomy. Bone loss after ovariectomy was more extensive in the 4th lumbar spine compared to the femur. Bone loss in the 4th lumbar spine was mainly caused by trabecular thinning, but in the femur, it was mainly caused by trabecular elimination.
Osteoporosis is a chronic disease of the skeleton caused by an imbalance between bone resorption and bone formation6,13,16). Currently, the incidence of osteoporosis is increasing rapidly in the elderly population, especially in postmenopausal women17).
Although no single animal model precisely mimics the human condition of osteoporosis, ovariectomized (OVX) animal models are the most frequently used model for studying postmenopausal osteoporosis with estrogen deficiency3,4,8,10-12,18,19). It is well known that, in ovariectomized animals, cortical bone loss does not match that of trabecular bone4,12,18). In a recent study by Ferretti et al.4), bone mass started to decrease earlier and more extensively in trabecular bone than in cortical bone. On the other hand, Thompson et al.18) reported that the pattern of bone loss was different between the spine and the long bone; the former lost bone by thinning trabeculae but the latter by eliminating trabeculae.
Many studies have focused on the histomorphometric changes and the change of bone turnover rates in the OVX rat model4,6-8,13-16); however, few mention the differences in histomorphometric changes between the spine and the long bone in the OVX rats.
Thus, in the present study, we performed histomorphometric analyses of the lumbar spine and the femur using micro-computed tomographic (CT) scans. Additionally we evaluated the changes of bone turnover rates 4 and 8 weeks after ovariectomy in rats.
All animal experiments were done in accordance with the animal care guidelines issued by the National Institute Health, and were approved by the Institutional Animal Care Committee at our university.
Twenty-four female Sprague-Dawley rats (11 weeks old) were purchased from Samtako Bio Inc. (Osan, Korea) and acclimated to conditions for 1 week before the experiment. Animals were housed in an air-conditioned room (relative humidity 45-65%) under a 12-h light/dark cycle at 22±20℃ and given free access to food and tap water.
Acclimated rats were assigned to one of two groups. The Sham group (n=12) underwent a sham operation at 12 weeks of age, and the OVX group (n=12) underwent bilateral ovariectomy to induce osteoporosis, at 12 weeks of age. We used the double dorso-lateral approach technique described in detail by Park et al.14) The sham controls underwent the same surgical procedure, but ovariectomy was not performed. Four weeks after OVX or sham operation, six rats from each of the two groups were euthanized, and the 4th lumbar spine and the femur were removed and blood samples were collected by cardiac puncture for serum isolation. Eight weeks after OVX or sham operations, the remaining rats in each group were euthanized, and the 4th lumbar spine and the femur were removed and blood samples were collected by cardiac puncture for serum isolation.
Body weights were checked once a week throughout the 8-week experiment period. Serum was separated by centrifugation (at 1500×g) and then stored at -80℃ until required for bone metabolic marker assays. Serum estrogen (estradiol, E2) was determined using an estradiol ELISA kit (ELISA, DRG instruments GmbH, Germany). In addition, the 4th lumbar vertebrae and the femur were removed, fixed in a 3.7% formaldehyde in phosphate-buffered saline solution (pH 7.4) for 16 h and then stored (4℃) in 80% ethanol for bone mass measurements.
Serum osteocalcin levels and alkaline phosphatase (ALP) activities (both sensitive biochemical markers of bone formation) were determined using osteocalcin EIA kits (Nordic Bioscience Diagnostics, Herlev, Denmark) and QuantiChrome ALP assay kits (DALP-250, BioAssay Systems, CA, USA), respectively. Serum levels of C-terminal telopeptide fragment of type I collagen C-terminus (CTX), which is generated by the osteoclast and is a marker of bone resorption, were determined using Rat-Laps ELISA kits (Nordic Bioscience Diagnostics)6,9).
Bone histomorphometric parameters and the microarchitectural properties of the 4th lumbar vertebrae and the femur were determined using a micro-CT system (eXplore Locus SP, GE Healthcare). The scanning protocol was set at X-ray energy settings of 80 kV and 80 µA, and the samples were scanned over one entire 360° rotation, with an exposure time of 3000 ms/frame. An isotopic resolution of 15-40 µm voxel size that displayed the microstructure of the rat femur and lumbar vertebra was selected, and the angle of increment around the sample was set to 0.40, which resulted in the acquisition of 900 2D images. A modified Feldkamp cone-beam algorithm was used for 3D reconstruction.
For the bone analysis, bone tissues, the region of the entire 4th lumbar vertebrae and of 2-6 mm from the growth plate of the femur were selected as the region of interest. Image information was obtained based on the automatic domain values produced by the computer. Bone mineral densities (BMD), trabecular bone volume fractions (BV/TV, %), trabecular thicknesses (Tb.Th.), trabecular numbers (Tb.N.), trabecular separations (Tb.Sp.), and cortical bone mineral densities (Cr.BMD) were used for the quantitative analysis, which was performed using 2.0+ ABA Microview software provided with the micro-CT system15,16).
All statistical comparisons were made using SPSS 17.0. Data are expressed as means + standard deviations. Repeated measure one-way analysis of variance was used to compare body weights and serum bone turnover markers in the OVX and sham groups. The two-sample t test was used to identify significant differences between the groups, and p values of ≤0.05 were considered significant.
Body weights were checked once a week throughout the 8-week experiment period. Fig. 1 shows changes in mean body weights of the twelve rats euthanized 8 weeks after OVX or sham operations. At the start of the experiment, body weights were similar in the two groups. However, at 4 weeks after surgery, the mean body weight in the OVX group was significantly greater than in the sham group, and this significance was maintained throughout the experiment period (p<0.05 at 4, 6, and 8 weeks, respectively).
As shown in Fig. 2, 4 and 8 weeks after surgery, serum estradiol levels were significantly lower in the OVX group (p=0.004 and p=0.000, respectively). Additionally, in the OVX group, the serum estradiol level at 8 weeks was significantly lower than at 4 weeks (p=0.001).
Fig. 3A and 3B summarize the serum levels of the bone formation biochemical markers, osteocalcin and ALP, and Fig. 3C shows CTX-1 serum levels, which is sensitive marker of bone resorption. Compared with the sham group, the OVX group had on average 31.2% and 83.8% higher osteocalcin levels at 4 and 8 weeks, which were statistically significant (p=0.010 and p=0.000, respectively). Additionally in the OVX group, the serum osteocalcin level at 8 weeks was significantly higher than at 4 weeks (p=0.002).
Compared with the sham group, the OVX group had on average 34.1% and 60.1% higher ALP levels at 4 and 8 weeks, which were statistically significant (p=0.0002 and p=0.000, respectively). Further, in the OVX group, the serum ALP level at 8 weeks was higher than at 4 weeks, but this difference did not reach statistical significance (p=0.056).
Compared with the sham group, the OVX group had on average 40.7% and 88.1% higher CTX-1 levels at 4 and 8 weeks, which were statistically significant (p=0.000 and p=0.003, respectively). The serum CTX-1 level in the OVX group at 8 week was also significantly higher than at 4 weeks (p=0.009).
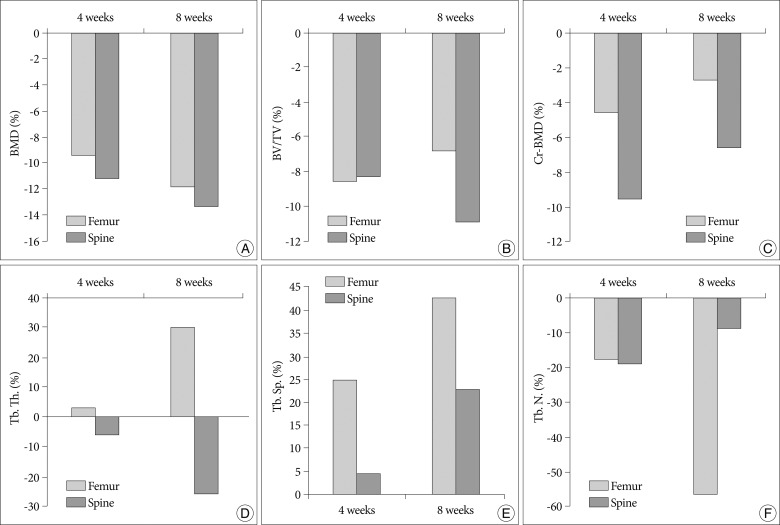
As shown in the representative micro-CT images of the 4th lumbar spine and the femur (Fig. 4), OVX rats 8 weeks after ovariectomy had fewer trabecular bone structures than at baseline and 4 weeks after ovariectomy. Fig. 5 shows the changes of BMD, BV/TV, Cr.BMD, Tb.Th., Tb.Sp., and Tb.N. of the 4th lumbar vertebra and the femur at 4 weeks and 8 weeks after ovariectomy. All values represent 100% minus the percentage of the value of the sham group/the value of the OVX group.
In the analyses of micro-CT scans of 4th lumbar vertebrae, compared with the sham group, the OVX group showed significant reductions in BMD at 4 and 8 weeks of 11.2% and 13.4%, respectively and an 8.3% and 10.9% reduction in BV/TV at 4 and 8 weeks after OVX, respectively. Furthermore, CrBMD in the OVX group was 9.6% and 6.6% lower than in the sham group at 4 and 8 weeks after ovariectomy, respectively. In addition, 8 weeks after ovariectomy, OVX rats showed a significant decrease in Tb.Th. (p=0.011) and a significant increase in Tb.Sp. (p=0.006) when compared to the sham group. However, Tb.N. in the OVX group was 9.1% lower than that of the sham group, which was not significantly different (p>0.05).
In the analyses of micro-CT scans of the femur, compared with the sham group, the OVX group showed a 9.6% and 11.9% reduction in BMD and an 8.6% and 6.8% reduction in BV/TV at 4 weeks and 8 weeks after ovariectomy, respectively. Furthermore, CrBMD in the OVX group was 4.6% and 2.7% lower than in the sham group at 4 and 8 week, respectively. In addition, 8 weeks after ovariectomy, OVX rats showed a significant increase in Tb.Th. (p=0.000) and a significant increase in Tb.Sp. (p=0.000) when compared to the sham group. However, Tb.N. 8 weeks after ovariectomy in the OVX group were 56.9% lower than that of the Sham group, which was significantly different (p=0.000).
It is well known that the incidence of osteoporosis is increasing rapidly in the elderly population, especially in postmenopausal women1,5,17). Adachi et al.1) reported that up to half of all postmenopausal women and an equal number of men are osteopenic.
Animal models of osteoporosis are essential for anticipating a treatment's efficacy and safety as measured by bone quality6,9,13,14,16,17). A recognized animal model of postmenopausal osteoporosis is the ovariectomized, mature rat5,8,10-12,18,19).
In our study, body weight in ovariectomized rats, increased by about 24% after 4 weeks following ovariectomy and remained elevated throughout the experiment period, which was comparable to the study by Omi et al.12) They described that weight gain efficiency (body weight gain/food intake) in their OVX group was significantly greater than in the sham group.
It is well known that the currently accepted mechanism of skeletal bone loss after postmenopausal osteoporosis is estrogen deficiency, which causes an imbalance in bone turnover, in which, bone resorption exceeds bone formation. In our study, as expected, 4 and 8 weeks after ovariectomy, serum estradiol levels were significantly lower in the OVX group compared with the sham group. Additionally in the OVX group, the percentage of serum estradiol decrease was 12.8% at 4 weeks and 28.3% at 8 weeks after ovariectomy. Our results support the presence of ovarian deficiency in the OVX model after bilateral ovariectomy.
Many studies have reported that estrogen deficiency increases serum osteocalcin, ALP, and CTX levels, which indicate increased bone turnover3,4,6,12,13,16,17). In the present study, ovariectomized rats were found to have higher osteocalcin, ALP and CTX levels than sham controls, indicating increased bone turnover due to menopause-induced estrogen deficiency, which is entirely consistent with previous studies4,12,17). In our OVX group, serum levels of osteocalcin, ALP, and CTX 8 weeks after ovariectomy were significantly higher than those after 4 weeks, which indicate an increased bone turnover rate at 8 weeks compared with that of 4 weeks. This was also consistent with previous studies2,8). Lei et al.8) reported that a typical osteoporotic profile was seen in bone marrow eight weeks after OVX. Therefore, when evaluating antiresorptive drugs or procedures, an ovariectomized rat model 8 weeks after OVX would be a better model for measuring BMD and other bone-related variables.
In our study, micro-CT scans of the 4th lumbar vertebra and the femur showed a significant decrease in BMD 4 weeks after ovariectomy and a marked decrease at 8 weeks, which supported the notion that our model successfully mimics the effects of osteoporosis.
It is well known that, in ovariectomized animals, cortical bone loss does not match that of trabecular bone4,12,18). Ferretti et al.4) reported that bone mass started to decrease earlier and more extensively in trabecular bone than in cortical bone. We observed similar findings in the present study (Fig. 5). Although BMD and BV/TV of the 4th lumbar vertebra and the femur were significantly decreased 4 and 8 weeks after ovariectomy, the extent of the decrease was greater in the 4th lumbar vertebra, which mainly consists of trabecular bone. However, CrBMD of the femur, which mainly consists of cortical bone, was less significantly decreased at 4 and 8 weeks after ovariectomy compared with that of the lumbar spine.
In the study of the FDA guidelines and animal models for osteoporosis, the pattern of bone loss was different between the spine and the long bone18). Thompson et al. investigated the loss of cancellous bone between the proximal tibia and lumbar spine. In their analyses of ovariectomized rats, the number of trabeculae significantly decreased after surgery, whereas trabecular thickness was only slightly affected in proximal tibiae. On the other hand, in lumbar vertebrae, the number of trabeculae was only slightly affected, but trabecular thinning was significantly decreased. Thus, they concluded that vertebrae lost bone by thinning trabeculae and tibiae lost bone by eliminating trabeculae. In the present study of ovariectomized rats, our results concurred with the findings of Thomson et al. We observed that trabecular separations were also significantly increased in both the lumbar spine and the femur 8 weeks after ovariectomy (22.9% and 42.8%, respectively). However, in the 4th lumbar spine, the increase of trabecular separation was mainly caused by thinning (26.1%) instead of a decrease in the number of trabeculae (9.1%). On the contrary, in the femur, the increase of trabecular separation was mainly caused by the decrease in the number of trabeculae (56.9%) instead of trabecular thinning.
The present study demonstrates different patterns of bone loss between the 4th lumbar spine and the femur in ovariectomized rats. Bone loss after ovariectomy was more extensive in the 4th lumbar spine compared with the femur. Additionally, bone loss in the 4th lumbar spine was mainly caused by trabecular thinning, but in the femur, it was mainly caused by trabecular elimination.
References
1. Adachi JD, Loannidis G, Berger C, Joseph L, Papaioannou A, Pickard L, et al. The influence of osteoporotic fractures on health-related quality of life in community-dwelling men and women across Canada. Osteoporos Int. 2001; 12:903–908. PMID: 11804016.

2. Bauss F, Dempster DW. Effects of ibandronate on bone quality : preclinical studies. Bone. 2007; 40:265–273. PMID: 16996333.

3. Canpolat S, Tug N, Seyran AD, Kumru S, Yilmaz B. Effects of raloxifene and estradiol on bone turnover parameters in intact and ovariectomized rats. J Physiol Biochem. 2010; 66:23–28. PMID: 20428990.

4. Ferretti M, Bertoni L, Cavani F, Zavatti M, Resca E, Carnevale G, et al. Influence of ferutinin on bone metabolism in ovariectomized rats. II : role in recovering osteoporosis. J Anat. 2010; 217:48–56. PMID: 20492429.

5. French DL, Muir JM, Webber CE. The ovariectomized, mature rat model of postmenopausal osteoporosis : an assessment of the bone sparing effects of curcumin. Phytomedicine. 2008; 15:1069–1078. PMID: 18693096.

6. Kim TH, Jung JW, Ha BG, Hong JM, Park EK, Kim HJ, et al. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J Nutr Biochem. 2011; 22:8–15. PMID: 20233653.

7. Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int. 1985; 37:594–597. PMID: 3937580.

8. Lei Z, Xiaoying Z, Xingguo L. Ovariectomy-associated changes in bone mineral density and bone marrow haematopoiesis in rats. Int J Exp Pathol. 2009; 90:512–519. PMID: 19765105.

9. Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, Nasti A, et al. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004; 145:3115–3121. PMID: 15059958.

10. Miller SC, Bowman BM, Jee WS. Available animal models of osteopenia--small and large. Bone. 1995; 17:117S–123S. PMID: 8579907.
11. Mosekilde L. Assessing bone quality--animal models in preclinical osteoporosis research. Bone. 1995; 17:343S–352S. PMID: 8579937.
12. Omi N, Ezawa I. The effect of ovariectomy on bone metabolism in rats. Bone. 1995; 17:163S–168S. PMID: 8579912.

13. Palumbo C, Ferretti M, Bertoni L, Cavani F, Resca E, Casolari B, et al. Influence of ferutinin on bone metabolism in ovariectomized rats. I : role in preventing osteoporosis. J Bone Miner Metab. 2009; 27:538–545. PMID: 19333679.

14. Park SB, Lee YJ, Chung CK. Bone mineral density changes after ovariectomy in rats as an osteopenic model : stepwise description of double dorso-lateral approach. J Korean Neurosurg Soc. 2010; 48:309–312. PMID: 21113356.

15. Sheng ZF, Dai RC, Wu XP, Fang LN, Fan HJ, Liao EY. Regionally specific compensation for bone loss in the tibial trabeculae of estrogen-deficient rats. Acta Radiol. 2007; 48:531–539. PMID: 17520429.

16. Shiraishi A, Miyabe S, Nakano T, Umakoshi Y, Ito M, Mihara M. The combination therapy with alfacalcidol and risedronate improves the mechanical property in lumbar spine by affecting the material properties in an ovariectomized rat model of osteoporosis. BMC Musculoskelet Disord. 2009; 10:66. PMID: 19527501.

17. Szulc P, Delmas PD. Biochemical markers of bone turnover : potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int. 2008; 19:1683–1704. PMID: 18629570.

18. Thompson DD, Simmons HA, Pirie CM, Ke HZ. FDA Guidelines and animal models for osteoporosis. Bone. 1995; 17:125S–133S. PMID: 8579908.

19. Turner AS. Animal models of osteoporosis--necessity and limitations. Eur Cell Mater. 2001; 1:66–81. PMID: 14562261.
Fig. 1
Graph showing temporal changes of body weights of the twelve rats euthanized 8 weeks after ovariectomy or sham operations. Values represent mean±standard deviations (n=12). *indicates p<0.05 for OVX vs. sham group comparisons. OVX: ovariectomized.
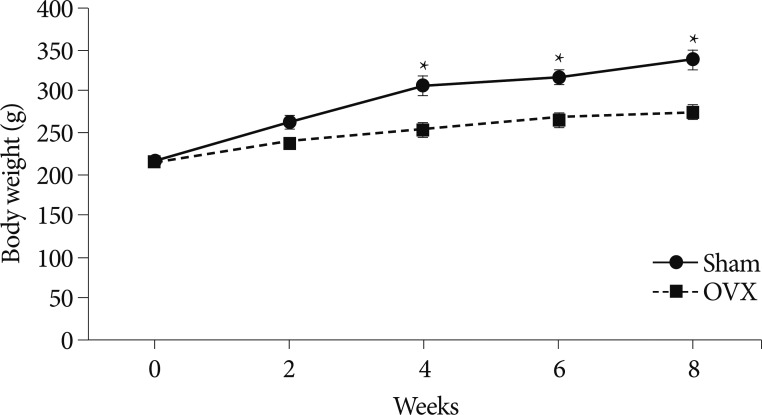
Fig. 2
Serum estrogen levels in the two study groups at 4 and 8 weeks after ovariectomy or sham operations. *indicates p<0.05 for 4 weeks vs. 8 weeks in the OVX group. OVX: ovariectomized.

Fig. 3
The serum osteocalcin (A) and ALP (B) concentrations were used as markers of bone formation. Type I collagen C-telopeptide (CTX) (C) concentration was used as a marker of bone resorption. Values are mean±standard deviations. *indicates p<0.05 for 4 weeks vs. 8 weeks in the OVX group. ALP: alkaline phosphatase, OVX: ovariectomized.
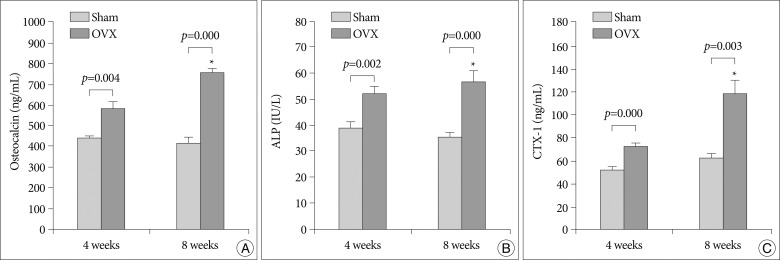
Fig. 4
Representative micro-CT images of the 4th lumbar spine (A) and the femur (B) in the OVX group. OVX rats 8 weeks after ovariectomy had less trabecular bone than at baseline and 4 weeks after ovariectomy. OVX: ovariectomized, CT: computed tomography.
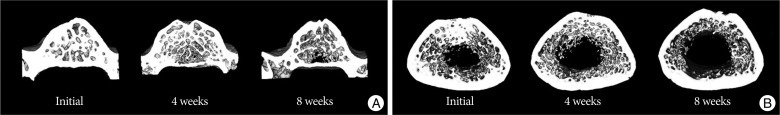
Fig. 5
Bar graph showing the result of histomorphometric analyses of 4th lumbar vertebrae in the sham and OVX groups at 4 and 8 weeks post-operation: bone mineral density (BMD) (A), trabecular bone volume fraction (BV/TV) (B), cortical bone mineral density (CrBMD) (C), trabecular thickness (Tb.Th.) (D), trabecular separation (Tb.Sp.) (E), and trabecular number (Tb.N.) (F). All values represent 100% minus the percentage of the value of the sham group/the value of the OVX group. OVX: ovariectomized.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download