INTRODUCTION
Although malignant tumors often metastasize to the brain, metastases to the pituitary gland are uncommon and constitute only 3-5% of all cases; primary sites usually include the lung, breast, and gastrointestinal tract
13,
14). Metastases occur in the 6th or 7th decade of life and this age range does not differ according to gender
13,
14). The most common symptom in patients with pituitary metastases, unlike those with primary pituitary tumors, is central diabetes insipidus (DI), followed by anterior hypopituitarism, visual loss, and central nervous system (CNS) disorders
8,
12). However, it is difficult to diagnose pituitary metastases, as the symptoms are nonspecific and the radiological differences from primary tumors are trivia
l5). Although differentiating whether a pituitary tumor is primary or secondary is crucial in making a treatment plan, it is difficult to make a diagnosis without pathological confirmation. If a tumor in the pituitary gland is confirmed to be metastatic, local tumor control is planned to relive symptoms, and the prognosis depends on the site of the primary malignancy
8,
12).
In South Korea, pituitary metastases from hard palate adenocarcinoma, periampullary adenocarcinoma, renal cell carcinoma, malignant lymphoma, and lung carcinoma have been reported. In addition, a case of central DI and panhypopituitarism due to pituitary metastasis from breast adenocarcinoma has been reported, but rarely confirmed pathologically
7,
11,
15).
Here, we report a pituitary mass found in a patient after surgery and chemotherapy for breast cancer. The patient underwent a surgical tumor removal via sublabial transsphenoidal approach and metastasis from breast invasive ductal adenocarcinoma was confirmed pathologically. This case is presented with a literature review.
Go to :

CASE REPORT
A 65-year-old female was referred to the Department of Neurosurgery from the Department of General Surgery with progressive bitemporal hemianopsia and loss of visual acuity in the left eye for three months. She had undergone a left radical mastectomy for invasive ductal carcinoma five years earlier and received four cycles of adjuvant chemotherapy using adriamycin/cyclophosphamide (AC) and radiation therapy. Twenty-eight months postoperatively, there was local left thoracic wall recurrence, and she underwent four additional cycles of docetaxel chemotherapy. Nine months later, after she developed new back pain, a bone scan and spine magnetic resonance imaging (MRI) were performed, which showed multiple metastases of breast cancer to the 4th and 5th lumbar vertebrae, right sacrum, and pelvis. The patient received radiation therapy to the metastases ten times and was prescribed letrozole. She had also been taking medication for hypertension.
Due to progressive left visual loss, brain MRI was performed. A 1.1×2.2×2 cm pituitary mass was found, with intermediate signal intensity on T1- and T2-weighted images and a well-enhancing nature. The mass was dumbbell shaped, compressed the optic chiasm, and displaced the pituitary stalk to the left (
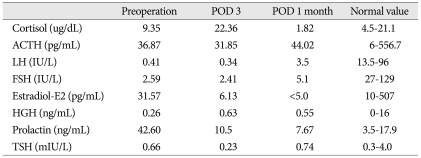
Fig. 1). Blood and urine tests were all normal, except for elevated prolactin measuring 42.60 ng/mL (
Table 1). On visual field examination, bitemporal hemianopsia was noted with decreased visual acuity on the left side.
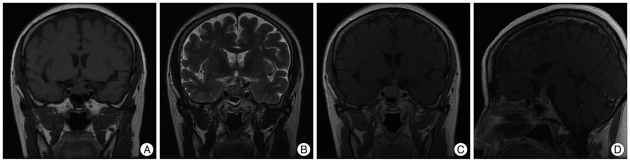 | Fig. 1Preoperative coronal view of T1 weighted image of sellar MRI (A), coronal view of T2 weighted image (B), coronal view of T1 Gadolinum enhanced image (C), sagittal view of T1 Gadolinum enhanced image (D). Images show the dumbbell shape pituitary mass with T1 intermediate, T2 intermediate and well enhancing nature, compressing the optic chiasm and invading the pituitary stalk. 
|
Table 1

Although the patient had a history of multiple metastases from breast cancer, the MRI findings and laboratory results suggested that the pituitary mass was a primary macroadenoma; therefore, we performed a surgical tumor removal via sublabial-transsphenoidal approach. Since the intraoperative findings of the mass were similar to a benign pituitary macroadenoma, no frozen pathological examination was performed (
Fig. 2).
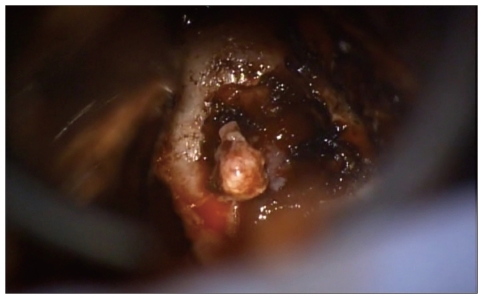 | Fig. 2Pituitary mass during the operation. The contents and colors were just similar with pituitary adenoma. 
|
The patient's postoperative course was uneventful and the prolactin level determined three days postoperatively was 10.50 ng/mL, which was within the normal range (
Table 1). Seven days postoperatively, her urine output increased to 130 mL hourly. A water restriction test was performed, and she was diagnosed with central DI. After administering vasopressin, the central DI improved and the left visual acuity improved both subjectively and objectively, from 0.16 preoperatively to 0.20 postoperatively.
The pathology report seven days postoperatively showed moderately differentiated adenocarcinoma; immunohistochemical staining with MOC-31 was positive. The final diagnosis was that the tumor was metastasized from breast invasive ductal carcinoma (
Fig. 3).
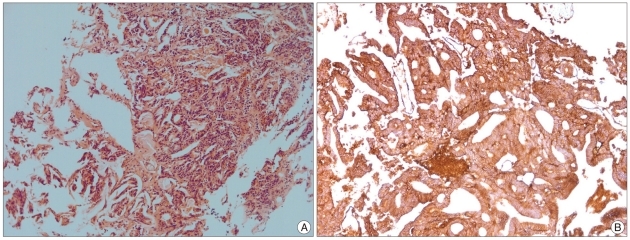 | Fig. 3Microscopic finding (HE, ×100). Characteristic findings of adenocarcinoma consist with invasive proliferation of cancer cell foci, formation of ductal structures by cancer cells were seen (A), immunohistochemical staining with MOC-31, which is a marker for adenocarcinoma showed positive (B). 
|
The patient underwent CyberKnife treatment for the remnant mass in the pituitary gland one month after the initial operation. Central DI occurred one week after the CyberKnife treatment, but improved with vasopressin. Following the CyberKnife treatment, the patient's hormone levels were within the normal ranges, so the vasopressin replacement was maintained (
Table 1). The patient returned for follow-up five months after the initial surgery and four months after the CyberKnife surgery, with no signs of metastasis. Subsequently, she was lost to follow-up.
Go to :

DISCUSSION
Metastatic spread of cancer to the pituitary gland is uncommon, but may occur more commonly than pituitary adenoma, invaoccurring in 3-5% of the patients with carcinoma
14). In females, the most likely source of pituitary metastasis is the breast, while in men, metastases tend to be from the lung
14). Two thirds of such tumors originate from these primary sites. In addition, gastrointestinal tract, prostate, kidney, thyroid, and pancreas primary tumors are possible sources of pituitary metastases
13). In 53-100% of cases, the primary sites are discovered simultaneously with the pituitary metastases both clinically and radiologically
8).
The routes of metastasis to the pituitary gland include hematogenous spread or via direct invasion through the skull base. The former is the main pathway. The most commonly involved site is the posterior lobe of the hypophysis (69-79%), followed by the anterior hypophysis, both the anterior and posterior hypophysis, and the stalk
13). Previous studies have reported that DI is common when the posterior hypophysis is involved, and anterior hypophysis dysfunction, cranial nerve dysfunction, headache, and hyperprolactinemia can occur
4,
8,
12). DI develops with involvement of the hypophysis, where antidiuretic hormone (ADH) is produced, or the pituitary stalk, where ADH is delivered. Since DI is a very rare symptom in pituitary adenoma, manifesting in less than 1%, it is the most important clinical manifestation differentiating a metastatic tumor from a primary pituitary adenoma
5,
10). However, in a series of 52 patients with pituitary metastasis, Heshmati et al.
3) found that visual impairment (i.e., a visual field defect or extraocular palsy) was the presenting complaint in 56% of the subjects. DI signaled pituitary involvement in 40% of the patients in their series
3). In our case, the patient's initial complaint was of a visual field defect, with no hormonal dysfunction; therefore, we presumed that the pituitary mass was a primary adenoma.
Although radiological diagnostic modalities have been developed, computed tomography (CT) is still insufficient for differentiating benign and malignant masses. An MRI study revealed that findings such as a loss of high signal intensity in the posterior hypophysis in T1-weighted images or hypertrophy of the pituitary stalk suggest metastases
9). In a meta-analysis of 70 cases with pituitary metastasis, Komninos et al.
5) reported that MRI may demonstrate an isointense or hypointense mass on T1-weighted images with a high-intensity signal on T2-weighted images, homogeneous enhancement with gadolinium and an absent high-signal intensity for the posterior lobe on T1-weighted images, a rapid increase of a sellar tumor with aggressive infiltration of adjacent tissues, or a dumbbell-shaped intrasellar and suprasellar tumor. Based on this report and our findings, a well-enhancing pituitary mass with stalk displacement suggests pituitary metastasis.
Although there have been several reports on sellar metastasis in South Korea, most of the cases presented with extensive involvement in adjacent tissues or multiple brain metastasis
7,
15). Diagnoses of pituitary metastases with those radiological findings and previous history of malignancy are not troublesome. However, findings in present case were more similar with those of primary adenoma, as the first symptom was bitemporal hemianopsia rather than hormonal symptoms, and radiologic finding was a single dumbell-shaped pituitary mass rather than multiple brain metastasis or extensive involvement of adjacent tissues. Therefore, physician should bear in mind the additional historical information to suspect pituitary metastasis, such as rapid onset and progression of symptom, unsuccessful treatment with bromocriptine, increased age and history of malignancy
15).
The treatment for pituitary metastasis is usually conservative, ranging from surgical removal, radiation surgery, or systemic chemotherapy, focusing on the extent and condition of the primary tumor. When visual symptoms are present, as in our case, surgical intervention can improve the symptoms
1,
12).
Nevertheless, survival does not differ between groups undergoing surgical intervention to remove the metastatic mass versus no surgery
8). That is, the prognosis of pituitary metastasis depends mainly on the extent of the primary tumor, rather than the mass in the pituitary gland. The reported life expectancy ranges from 6 to 22 months. In cases with pituitary stalk invasion, the prognosis is usually poorer, i.e., 2-4 months
2,
6).
In this case, the MRI findings and primary symptoms were not enough to differentiate the sellar metastasis from primary pituitary adenoma. The ophthalmologic symptoms improved after surgical and CyberKnife treatment for the pituitary metastasis. Although surgical intervention does not improve life expectancy, this case proved that surgery can improve the symptoms caused by pituitary metastasis, as previously reported
15).
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download