Abstract
We present the rare case of solitary xanthogranuloma in the upper cervical column mimicking a Brown-Sequard syndrome. A 29-year-old man complained with right hemiparesis and left hypoesthesia after a car accident. Computed tomography and magnetic resonance images revealed a lobulated homogenously well-enhancing mass in between posterior arch of the atlas (C1) and spinous process of the axis (C2) resulting in a marked spinal canal narrowing with cortical erosions. The patient was managed by complete resection of the tumor with partial laminectomy with lower half of C1 posterior arch and upper half of C2 spinous process. The authors advise complete removal of the xanthogranuloma and consideration as a differential diagnosis of lesions among upper cervical lesions.
Juvenile xanthogranuloma (JXG) is a proliferative histiocytic disorder of childhood and regarded as a non-Langerhans cell histiocytosis7). JXG is not a true neoplasm but rather a reactive proliferation of histiocytes and the etiology and pathogenesis are still unknown. The clinical features have been described as multiple, cutaneous and self-limited lesions occurring in children in their first 2 decades of life and, thus, have been called JXG9,14-17). However, JXG occurring as extracutaneous lesion especially involving an isolated spinal column is extremely rare in an adult and the incidence has never been reported. Herein, the authors describe the unique case of a 29-year-old man harboring an isolated epidural C1 to C2 xanthogranuloma and treated with total surgical resection through partial laminectomy for the decompression of spinal canal and the maximal preservation of normal structures to maintain the cervical motion without instrumental fixation.
A 29-year-old male patient presented with right side weakness and left side numbness that occurred just after a car accident. Upon physical examination, there was no abnormal finding, except for tenderness in the upper cervical area. Routine laboratory tests demonstrated normal results. Motor weakness of right side extremity was disclosed as 1/5 for arm and 2/5 for leg with slightly hyperactivity of deep tendon reflex. Sensory examination showed significantly decreased pain, temperature, vibration, and discriminatory touch sensations below the left C2 dermatome. Babinski's reflex and Hoffman's sign were present on the left side. Based on the symptoms and neurological findings, the assumptive diagnosis of Brown-Sequard syndrome was made. The patient had no symptoms or past medical history related to a cervical spine lesion before the trauma. Cervical plain films revealed straightening of the cervical spine with loss of normal lordosis and widening between the posterior arch of the atlas and spinous process of the axis. A computed tomography (CT) scan of the upper cervical spine revealed a large round mass which measured 25×18×24 mm with relatively homogeneous strong enhancement and was located between the C1 and C2 interspinous space. In addition, the CT scan showed cortical osseous erosions at the lower C1 posterior arc and upper C2 spinous process. Cervical magnetic resonance images (MRI) revealed an epidural dumbbell-shaped mass traversing the C1 to C2 interspinous space, compressing the surrounding structures (Fig. 1). The spinal cord was compressed by the lesion which was isointense on T1-weighted image (WI) and mixed hypointense on T2WI with homogenous strong enhanced after gadolinium administration. Radiologic analysis indicated possible lymphoma, meningioma, schwannoma or other neuronal tumors.
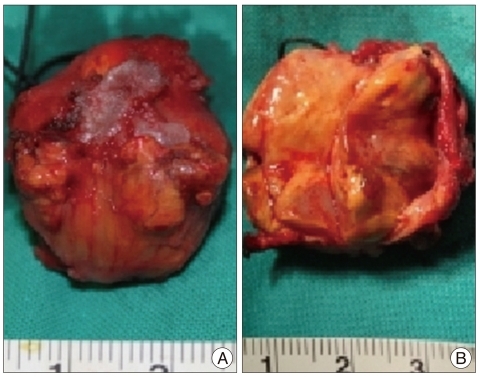
The patient underwent a surgery immediately for decompression of the spinal cord. A total tumor resection was performed with only a partial laminectomy from the lower half of the C1 to upper half of C2 without any instrumentation and access to the tumor. After partial lamicectomy, an extradural and yellowish mass was exposed with well-demarcated from the surrounding structures but adhesive at the epidural area. Under microscopy, the tumor was delicately dissected, and en bloc removal was achieved (Fig. 2). The mass was ovoid, soft like gelatin, avascular, and well-encapsulated, measuring 25 mm at the largest diameter, and was adherented to the outer layer of the dura mater. And, there was no root invasion. On the basis of the clinical and operative features, we thought that the lesion originated from the outer aspect of the dura mater. Upon sectioning, the mass was particularly yellow and white with cystic components (Fig. 3).
After surgery, the patient's right hemiparesis fully recovered in 2 weeks. However, a slightly tingling numbness on his left arm remained and was controlled through medication in the outpatient clinic for 4 months. Currently, the patient has no symptoms without instability of cervical spine. Cervical MRI with gadolinium enhancement at 2 years after surgery demonstrated no recurrent lesion (Fig. 4).
Histological examination of the tumor uncovered focal aggregation cells with ample, clear, and foamy cytoplasm in a background of loose, fibrous stroma of xanthic appearance. Large, round cells had irregular vesicular nuclei which were regular and small with condensed chromatin (Fig. 4). The specimen was underwent to immunohistochemical testing, yielding strongly positive for CD 68 and lysozyme stain, which confirmed that the large foam cells were histiocytes. The test with CD-1a was negative, thus ruling out Langerhans' cell histiocytosis. GFAP stain and S-100 protein were negative. Thus, a diagnosis of xanthogranuloma was established.
In 1905, the first case of JXG was reported by Adamson1), who termed the entity a congenital xanthoma multiplex, and is not a true tumor, but rather a reactive proliferation of histiocytes. JXG is the most common form of non-Langerhans cell histiocytosis. The actual incidence of JXG is unknown but may be higher than is generally recognized, because JXGs occur early in life. A male predominance has been noted in childhood; however, in adults, there is no sex predilection. Most tumors appear early in life with 5% to 17% of JXGs present at birth and 40% to 70% appearing during the first year of life. However, despite the term JXG, onset during adulthood is possible, with a peak incidence in the late 20's to early 30's10). Autopsy series suggest the incidence of these lesions varies between 1.6% and 7% of the general 2012population3). CNS involvement in cases of cutaneous and/or extracutaneous JXG is rare8). Isolated CNS JXG can be classified according to its localization and the site of implantation in the following : 1) cerebral intraparenchymal, 2) dural, and 3) origination from the cranial or spinal column19). Solitary xanthogranuloma, especially involving the spine, is exceedingly rare4,16,18,19).
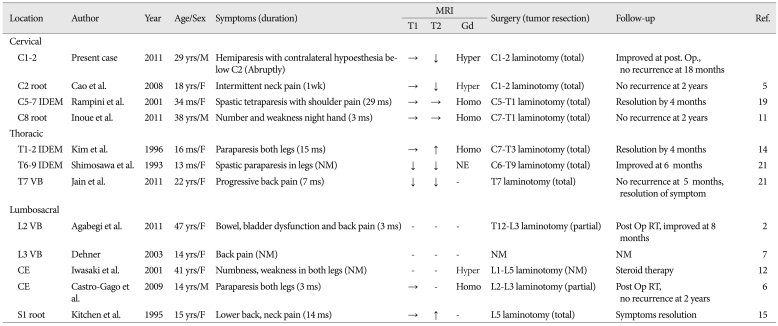
To date, 12 such cases are found reported in the English literature2,5-7,11-15,19,21). Four of these were in the cervical spine, three cases involved the thoracic and five were lumbosacral lesions. There has been a single prior report of JXG involving the upper cervical spine (C2 nerve root in a 18-year-old female)5). To the best of our knowledge, the authors of the current study present the second report of a solitary JXG involving upper cervical spine. And this is the second report of a solitary xanthogranuloma occurring in a male adult (although the prevalence is without sex distinction). Almost all cases of intradural-extramedullary JXG were seen in infants because of the early symptom developing due to cord compression by the lesion14,19,21). Paravertebral JXG was founded in relatively elder age than ordinary JXG or in adults (Table 1). The authors considered naming the tumor 'solitary xanthogranuloma', and excluding 'juvenile' because the tumor was discovered in an adult. Analyzing the reported 12 cases involving the spine, the clinical features were related with the anatomic localization, as in slow-growing tumors, and gradually exaggerated except in the present case. Interestingly, the patient in the current study abruptly presented symptoms similar to Brown-Sequard syndrome after a car accident. The patient had no symptoms before, possibly because the tumor was located at the epidural space of the C1 to C2 level, without compression of the spinal cord. However, after trauma, an acute compressive myelopathy developed from the mass effect of the tumor.
A MRI is the best method for the localization of tumors and their relationship to adjacent structures. The lesion may appear hypo-, iso-, and slightly hyperintense in T1WI and T2WI. Schultz et al.20) declared that spinal xanthogranuloma does not enhance, whereas cerebral xanthogranuloma does enhance homogenously with gadolinium. The tumor should be distinguished from other tumors of neural structure origin which arise from the nerve root ganglion or dura-mater (e.g. schwannoma, meningioma, neuroma, and lymphoma). Upon gross anatomic examination, the xanthogranuloma is a well-encapsulated and round lesion with a yellowish-to-grayish surface with or without cystic components. Xanthogranuloma is confirmed through pathological and immunohistochemical studies. Microscopically, there are foamy histiocytic cells with or without Touton giant cells, which can be found in a background of mononuclear cells, and spindle cells. In immunohistochemistry studies, xanthogranuloma has mononuclear cells, giant cells, and spindle cells which are positive for the lysozyme stain and CD68 but negative for CD1a and S-100 proteins, which are the reactive markers of Langerhans cells.
Currently, there is no standard treatment for solitary xanthogranuloma involving the CNS because of extremely low incidence. Moreover the tumors involving the spine may grow slowly without regression and make the symptoms gradually worsen. This characteristic is significantly distinct from the skin lesions which are spontaneous regression in natural course. Although there are only a few cases of spinal xanthogranuloma, as much as possible of the tumor should be removed, because the tumor is pathologically and clinically benign. Additionally, there is no report regarding a recurrent case of solitary CNS xanthogranuloma after gross total removal. Although there are some cases treated with chemotherapy or adjuvant therapy after partial resection, total resection with preservation of neural structures appears curative for patients. In the present case, the authors thought that, if possible, the tumor should be completely removed with preservation of normal structures and cervical motion. En bloc tumor resection was performed by minimal bone work, which included a partial hemilaminectomy at the lower half of the C1 and upper half of C2 without instrumentation or fixation. Finally, the patient's cervical motion could be preserved without instability and the symptoms relieved without complications. The patient should be followed for long-term and given medical observation after total resection, because the natural course of solitary CNS xanthogranuloma is unknown.
The authors report the second case of a solitary CNS xanthogranuloma involving the spine in a male adult. Surgeons should consider differential diagnosis for such tumors in the spine. The tumor must be removed as much as possible with preservation of the neural structure, because of the curative potential for patients and the tumor's benignity. In addition, the patient should be followed up long-term and evaluated for the possibility of remission and recurrence of the tumor.
References
1. Adamson NF. Congenital xanthoma multiplex in a child. Br J Dermatol. 1905; 17:222–223.
2. Agabegi SS, Iorio TE, Wilson JD, Fischgrund JS. Juvenile xanthogranuloma in an adult lumbar spine : a case report. Spine (Phila Pa 1976). 2011; 36:E69–E73. PMID: 21192217.
3. Ayres WW, Haymaker W. Xanthoma and cholesterol granuloma of the choroid plexus. Report of the pathological aspects in 29 cases. J Neuropathol Exp Neurol. 1960; 19:280–295. PMID: 13795372.

4. Boström J, Janssen G, Messing-Jünger M, Felsberg JU, Neuen-Jacob E, Engelbrecht V, et al. Multiple intracranial juvenile xanthogranulomas. Case report. J Neurosurg. 2000; 93:335–341.
5. Cao D, Ma J, Yang X, Xiao J. Solitary juvenile xanthogranuloma in the upper cervical spine : case report and review of the literatures. Eur Spine J. 2008; 17(Suppl 2):S318–S323. PMID: 18228052.
6. Castro-Gago M, Gómez-Lado C, Alvez F, Alonso A, Vieites B. Juvenile xanthogranuloma of the cauda equina. Pediatr Neurol. 2009; 40:123–125. PMID: 19135628.

7. Dehner LP. Juvenile xanthogranulomas in the first two decades of life : a clinicopathologic study of 174 cases with cutaneous and extracutaneous manifestations. Am J Surg Pathol. 2003; 27:579–593. PMID: 12717244.

8. Freyer DR, Kennedy R, Bostrom BC, Kohut G, Dehner LP. Juvenile xanthogranuloma : forms of systemic disease and their clinical implications. J Pediatr. 1996; 129:227–237. PMID: 8765620.

9. George DH, Scheithauer BW, Hilton DL, Fakhouri AJ, Kraus EW. Juvenile xanthogranuloma of peripheral nerve : a report of two cases. Am J Surg Pathol. 2001; 25:521–526. PMID: 11257628.
10. Hernandez-Martin A, Baselga E, Drolet BA, Esterly NB. Juvenile xanthogranuloma. J Am Acad Dermatol. 1997; 36:355–367. quiz 368-369. PMID: 9091465.

11. Inoue H, Seichi A, Yamamuro K, Kojima M, Kimura A, Hoshino Y. Dumbbell-type juvenile xanthogranuloma in the cervical spine of an adult. Eur Spine J. 2011; 20(Suppl 2):S343–S347. PMID: 21468645.

12. Iwasaki Y, Hida K, Nagashima K. Cauda equina xanthogranulomatosis. Br J Neurosurg. 2001; 15:72–73. PMID: 11303669.

13. Jain A, Mathur K, Khatri S, Kasana S, Jain SK. Rare presentation of juvenile xanthogranuloma in the thoracic spine of an adult patient : case report and literature review. Acta Neurochir (Wien). 2011; 153:1813–1818. PMID: 21626171.

14. Kim DS, Kim TS, Choi JU. Intradural extramedullary xanthoma of the spine : a rare lesion arising from the dura mater of the spine : case report. Neurosurgery. 1996; 39:182–185. PMID: 8805158.

15. Kitchen ND, Davies MS, Taylor W. Juvenile xanthogranuloma of nerve root origin. Br J Neurosurg. 1995; 9:233–237. PMID: 7632374.

16. Kraus MD, Haley JC, Ruiz R, Essary L, Moran CA, Fletcher CD. "Juvenile" xanthogranuloma : an immunophenotypic study with a reappraisal of histogenesis. Am J Dermatopathol. 2001; 23:104–111. PMID: 11285404.
17. Lesniak MS, Viglione MP, Weingart J. Multicentric parenchymal xanthogranuloma in a child : case report and review of the literature. Neurosurgery. 2002; 51:1493–1498. discussion 1498. PMID: 12445357.

18. Paulus W, Kirchner T, Michaela M, Kühl J, Warmuth-Metz M, Sörensen N, et al. Histiocytic tumor of Meckel's cave. An intracranial equivalent of juvenile xanthogranuloma of the skin. Am J Surg Pathol. 1992; 16:76–83. PMID: 1728198.
19. Rampini PM, Alimehmeti RH, Egidi MG, Zavanone ML, Bauer D, Fossali E, et al. Isolated cervical juvenile xanthogranuloma in childhood. Spine (Phila Pa 1976). 2001; 26:1392–1395. PMID: 11426158.

20. Schultz KD Jr, Petronio J, Narad C, Hunter SB. Solitary intracerebral juvenile xanthogranuloma. Case report and review of the literature. Pediatr Neurosurg. 1997; 26:315–321. PMID: 9485160.
21. Shimosawa S, Tohyama K, Shibayama M, Takeuchi H, Hirota T. Spinal xanthogranuloma in a child : case report. Surg Neurol. 1993; 39:138–142. PMID: 8351627.
Fig. 1
Preoperative MRI showing abnormal signal intensities on epidural dumbbell-shaped mass (measuring 25×18×24 mm) traversing the C1 to C2 interspinous space, compressing surrounding structures. The dumbbell-shaped lesion reveals isointense on sagittal T1-WI (A), mixed hypointense on T2-WI (B), and well-enhanced after gadolinium administration (C). The spinal cord was compressed at the C2 level, and the signal change appeared on T2-WI. MRI : magnetic resonance images, WI : weighted image.
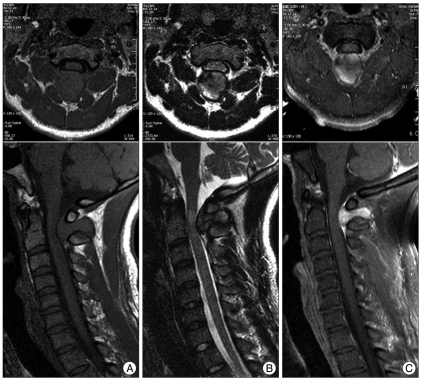
Fig. 2
Post-operative CT scan. Partial hemilaminectomy, from the lower half of the C1 posterior arch to upper half of C2 spinous process, was performed (A : mid-sagittal, B : 3-dimensional reconstruction). C : Follow-up MRI with enhancement after postoperative 2 years reveals no residual and no recurrence. CT : computed tomography, MRI : magnetic resonance images.
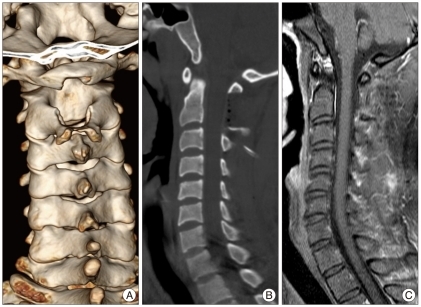
Fig. 3
Photomicrographs of the tumor reveals focal aggregation of cells with ample, clear, and foamy cytoplasm. There are large round cells with irregular vesicular nuclei. H&E, original magnification ×200.

Fig. 4
Gross finding of the tumor. A : External surface of the tumor; ovoid, yellowish, encapsulated with a white-colored adhesion scar at the mid-portion of the mass shown in the picture. B : Mid-section of the tumor, particularly yellow and white with cystic components.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download