Abstract
Although the mechanism of hemifacial spasm (HFS) is not yet well established, vascular compression of the facial nerve root exit zone and hyperexcitability of the facial nucleus have been suggested. We report a case of HFS in the setting of coinciding intracranial hemorrhage (ICH) of the pons and proximal ligation of the contralateral vertebral artery (VA) for the treatment of a fusiform aneurysm of the distal VA and discuss the possible etiologies of HFS in this patient. A 51-year-old male with an ICH of the pons was admitted to our hospital. Neuroimaging studies revealed an incidental fusiform aneurysm of the right VA distal to the origin of the posterior inferior cerebellar artery. Eight months after proximal ligation of the VA the patient presented with intermittent spasm of the left side of his face. Pre- and post-ligation magnetic resonance angiography revealed an enlarged diameter of the VA. The spasm completely disappeared after microvascular decompression.
Hemifacial spasm (HFS) is a movement disorder characterized by involuntary contractions of the facial muscles. Although the mechanism of HFS is not yet well established, vascular compression of the facial nerve root exit zone (REZ) is one likely mechanism given the effectiveness of microvascular decompression (MVD) in the treatment of the disorder1,7,9). Moreover, the vascular compression theory has been accepted as the etiology of rare secondary HFS in some cases of cerebellopontine angle (CPA) tumors, aneurysms, and arteriovenous malformations (AVMs)5,7,10,13,17,18). However, another theory for the mechanism of HFS, hyperexcitability of the facial nucleus, has also been suggested. This theory was inspired by various cases of brain stem gliomas, 4th ventricle tumors, brain stem hemorrhages, and lacunar infarctions without direct vascular compression2,6,21,22).
We present a case of HFS in the setting of coinciding brain stem hemorrhage and proximal ligation of the contralateral vertebral artery (VA) for the treatment of a fusiform aneurysm of the distal VA and discuss the possible etiologies of HFS in this patient.
A 51-year-old male presenting with abrupt onset drowsiness was admitted to our hospital. A neurological examination revealed mildly confused orientation, gaze limitation to the left side in both eyes, mild palsy of the left face, and right hemiparesis. The patient had a history of hypertension which was being medically managed. Brain computed tomography (CT) revealed a hematoma in the posterior portion of the lower pons with minimal mass effect (Fig. 1A). After 2 months of conservative management, his mental status and gaze limitation were completely recovered while the facial palsy and hemiparesis were improved but still remained.
Magenetic resonance angiography (MRA) was performed to evaluate for a possible lesion causing an intracranial hemorrhage (ICH); a fusiform aneurysm was found in the right VA. Although the aneurysm was located adjacent to the pontine hematoma, it appeared unlikely to be causing an ICH because the hematoma was totally encased within the pons and there was no evidence of subarachnoid hemorrhage on serial brain CT and MR. On angiography, the fusiform aneurysm was located distal to the origin of the posterior inferior cerebellar artery (Fig. 1B). Because there was no neurological deterioration during a thirty minute balloon test occlusion, we performed proximal ligation of the VA via a retromastoid suboccipital craniotomy. The procedure was performed without any complications and the patient was discharged and received physical therapy in local clinics.
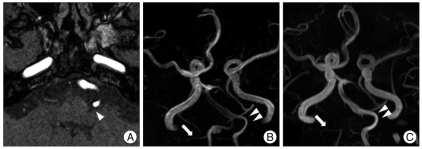
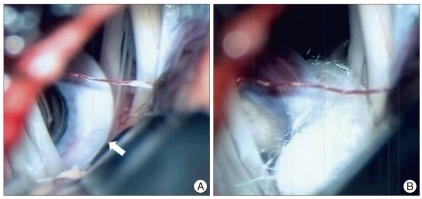
Eight months after the operation the patient visited our hospital complaining of involuntary left facial twitching. The spasm had initially begun in the left orbicularis oculi muscle during physical therapy. Despite two years of medication, the frequency of the spasms increased, the severity of the spasms increased, and the spasms spread to involve other ipsilateral facial muscles. During careful consideration of the cause of these spasms we identified a hemodynamic difference between the pre- and post-ligation MRAs. The left VA, which was compressing the facial nerve REZ, did not change architecturally but enlarged in diameter (Fig. 2). Although pontine ICH is also considered a potential cause of HFS, the long interval between the ICH and the onset of HFS prompted us to perform MVD in this patient. The facial nerve REZ was subsequently found to be compressed by an elongated VA (Fig. 3). Immediately after decompression the patient was free from symptoms and has not had a recurrence during 2 years of follow-up.
Although the mechanism of HFS is not yet clearly defined, vascular compression of the facial nerve REZ is generally considered the primary etiology of HFS9,11). Offending vessels are most often found on preoperative radiologic evaluation or during surgery; MVD of these offending vessels has yielded successful outcomes in large serial studies1,8). In several rare secondary causes of HFS, including CPA tumors, aneurysms, and AVMs, compression of the facial nerve REZ by altered vasculature is also considered the etiology of HFS5,7,10,13,17,18).
Another theory for the mechanism of HFS, hyperexcitability of the facial nucleus, has also been suggested15,20). Specifically, in cases of brain stem gliomas, 4th ventricle tumors, brain stem hemorrhages, and lacunar infarctions without vascular compression, facial nucleus hyperactivity has been suggested as the etiology of HFS2,6,21,22). Moreover, Chang et al.4) suggested that some unknown factors, in addition to vascular compression, may contribute to the etiology of HFS because HFS did not always resolve despite sufficient decompression. Previously, a case of pontine ICH secondary to head trauma presented with HFS without any vascular compression22). In this case, the HFS developed 2 days after the ICH; the HFS subsequently resolved with systemic steroid injection. Interestingly, our patient had both possible causes of HFS, pontine ICH and vascular compression.
Increased hemodynamic stress of the VA after contralateral VA occlusion has been suggested in several cases3,12,14). There have been two reported cases of de novo dissecting aneurysm of the VA that occurred 3 weeks and 5 months after balloon occlusion of the contralateral VA. Mizutani and Aruga14) reported a case of enlargement of a pre-existing VA dissecting aneurysm after contralateral VA occlusion which presented as facial spasm 6 years after VA occlusion.
A meaningful relationship between an increase in hemodynamic stress and the development of HFS has been suggested by some authors; two cases of VA aneurysms causing ipsilateral HFS resolved after proximal occlusion of the VA16,19). One case consisted of a VA dissecting aneurysm that was occluded by endovascular coil embolization and the other case consisted of a VA fusiform aneurysm treated with surgical ligation. In both cases the spasms disappeared immediately after proximal occlusion without any need for further treatment of the spasms. In our case, there was no development of a dissecting aneurysm in the contralateral VA after proximal VA ligation; however, preoperative- and 20-month postoperative-MRA demonstrated increased signal intensity and enlarged VA diameter, suggesting increased hemodynamic stress (Fig. 2B, C).
In our case, we had to consider both nucleus hyperexcitability from a pontine ICH and vascular compression by altered hemodynamics as potential causes of the HFS. The patient had not experienced spasm prior to the ligation, although the facial nerve REZ was affected by the VA on preoperative MRA. However, we ultimately decided to perform MVD because of the relatively long interval from the ICH to onset of the HFS and because a MRI performed after the onset of the HFS showed that the hematoma had already resolved. The spasm completely disappeared immediately after decompression of the offending VA. Therefore, we concluded that the increased hemodynamic stress on the left VA following contralateral VA occlusion had applied pressure on the facial nerve REZ and triggered the onset of the HFS in this patient.
To the best of our knowledge, this is an extremely rare and unique case of HFS. We suggest that hemodynamic changes themselves could be causative of HFS without pathological vascular alterations. Furthermore, even in cases where there are other possible causes of facial nucleus hyperexcitation, evaluation for vascular abnormalities should be performed.
References
1. Barker FG 2nd, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. J Neurosurg. 1995; 82:201–210. PMID: 7815147.

2. Bills DC, Hanieh A. Hemifacial spasm in an infant due to fourth ventricular ganglioglioma. Case report. J Neurosurg. 1991; 75:134–137. PMID: 2045898.
3. Caplan LR, Baquis GD, Pessin MS, D'Alton J, Adelman LS, DeWitt LD, et al. Dissection of the intracranial vertebral artery. Neurology. 1988; 38:868–877. PMID: 3368067.

4. Chang JW, Chang JH, Choi JY, Kim DI, Park YG, Chung SS. Role of postoperative magnetic resonance imaging after microvascular decompression of the facial nerve for the treatment of hemifacial spasm. Neurosurgery. 2002; 50:720–725. discussion 726. PMID: 11904021.

5. Choi SK, Rhee BA, Lim YJ. Hemifacial spasm caused by epidermoid tumor at cerebello pontine angle. J Korean Neurosurg Soc. 2009; 45:196–198. PMID: 19352486.

6. Elgamal EA, Coakham HB. Hemifacial spasm caused by pontine glioma : case report and review of the literature. Neurosurg Rev. 2005; 28:330–332. PMID: 16001287.

7. Han IB, Chang JH, Chang JW, Huh R, Chung SS. Unusual causes and presentations of hemifacial spasm. Neurosurgery. 2009; 65:130–137. discussion 137. PMID: 19574834.

8. Huh R, Han IB, Moon JY, Chang JW, Chung SS. Microvascular decompression for hemifacial spasm : analyses of operative complications in 1582 consecutive patients . Surg Neurol. 2008; 69:153–157. discussion 157. PMID: 18261641.

9. Jannetta PJ, Abbasy M, Maroon JC, Ramos FM, Albin MS. Etiology and definitive microsurgical treatment of hemifacial spasm. Operative techniques and results in 47 patients. J Neurosurg. 1977; 47:321–328. PMID: 894338.
10. Kobata H, Kondo A, Iwasaki K. Cerebellopontine angle epidermoids presenting with cranial nerve hyperactive dysfunction : pathogenesis and long-term surgical results in 30 patients. Neurosurgery. 2002; 50:276–285. discussion 285-286. PMID: 11844262.

11. Kondo A, Ishikawa J, Konishi T, Yamasaki T. [Mechanism of vascular compression of cranial nerves -- role of changes of vertebro-basilar vasculatures (author's transl)]. Neurol Med Chir (Tokyo). 1981; 21:287–293. PMID: 6166884.
12. Kubo Y, Miura K, Suzuki M, Tsuiki K, Kuwata N, Kubo N, et al. Development of a dissecting aneurysm on the vertebral artery immediately after occlusion of the contralateral vertebral artery : a case report. Neurosurg Rev. 1998; 21:177–180. PMID: 9795957.

13. Lee SH, Rhee BA, Choi SK, Koh JS, Lim YJ. Cerebellopontine angle tumors causing hemifacial spasm : types, incidence, and mechanism in nine reported cases and literature review. Acta Neurochir (Wien). 2010; 152:1901–1908. PMID: 20845049.

14. Mizutani T, Aruga T. [Rupture and outcome of vertebrobasilar dissecting aneurysms presenting subarachnoid hemorrhage]. Surg Cereb Stroke. 1994; 22:389–393.

15. Møller AR. Hemifacial spasm : ephaptic transmission or hyperexcitability of the facial motor nucleus? Exp Neurol. 1987; 98:110–119. PMID: 2820779.

16. Nagashima H, Orz Y, Okudera H, Kobayashi S, Ichinose Y. Remission of hemifacial spasm after proximal occlusion of vertebrobasilar dissecting aneurysm with coils : case report. J Clin Neurosci. 2001; 8:43–45. PMID: 11148077.

17. Nagata S, Matsushima T, Fujii K, Fukui M, Kuromatsu C. Hemifacial spasm due to tumor, aneurysm, or arteriovenous malformation. Surg Neurol. 1992; 38:204–209. PMID: 1440205.

18. Rhee BA, Kim TS, Kim GK, Leem WL. Hemifacial spasm caused by contralateral cerebellopontine angle meningioma : case report. Neurosurgery. 1995; 36:393–395. PMID: 7731520.

19. Sato K, Ezura M, Takahashi A, Yoshimoto T. Fusiform aneurysm of the vertebral artery presenting hemifacial spasm treated by intravascular embolization : case report. Surg Neurol. 2001; 56:52–55. PMID: 11546578.

20. Valls-Sole J, Tolosa ES. Blink reflex excitability cycle in hemifacial spasm. Neurology. 1989; 39:1061–1066. PMID: 2761700.

21. Vermersch P, Petit H, Marion MH, Montagne B. Hemifacial spasm due to pontine infarction. J Neurol Neurosurg Psychiatry. 1991; 54:1018. PMID: 1800652.

22. Wang HC, Lu CH, Lee RJ, Yang TM, Hung KS. Post-traumatic hemifacial spasm. J Clin Neurosci. 2006; 13:681–683. PMID: 16860719.

Fig. 1
A : An axial computed tomography scan without contrast medium on admission, shows a hematoma in the lower pons. B : Right lateral view of vertebral angiogram, shows a fusiform aneurysm of the right vertebral artery, distal to the origin of the posterior inferior cerebellar artery.
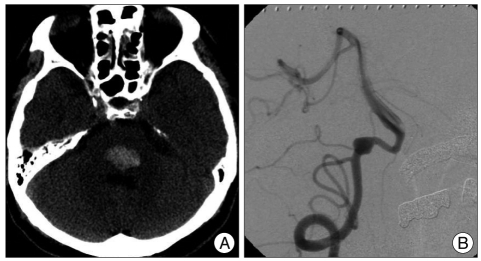
Fig. 2
A : A short range 3D magnetic resonance angiography (MRA). 7th nerve root exit zone compressed by the VA (arrowhead). Pre- (B) and 20-month post-ligation MRAs (C). The fusiform aneurysm is not visible postoperatively with intact patency of right PICA (arrows). Note enlargement in diameter of left VA after treatment (double arrowheads). VA : vertebral artery, PICA : posterior inferior cerebellar artery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download