Abstract
The intracranial stent functions primarily to prevent protrusion of coils into the parent vessel during the embolization of wide-necked cerebral aneurysms and might also reduce aneurysm recanalization rate. In spite of these advantages, little is known about the long-term interaction of the stent with the parent vessel wall. We present a rare case of severe in-stent stenosis occurring as a delayed complication of Neuroform stent-assisted coil embolization of an unruptured intracranial aneurysm.
Stents play an important role in management of cerebral aneurysms. A stent reconstructs the parent artery, assists coil embolization, and decreases flow activity within an aneurysm. The Neuroform stent (Boston Scientific, Natick, MA, USA) is the first microcatheter-delivered stent, designed specifically for the treatment of cerebral aneurysms5). Preliminary data suggest that the stent confers a considerable durability advantage, with high rates of both progressive thrombosis and stability observed at follow-up4). However, an in-stent stenosis may occur within the stented artery and compromises circulation at the parent artery3,6). The rate of angiographic or clinically significant in-stent stenosis after Neuroform stent-assisted aneurysm treatment is currently not characterized, but is thought to be very low11). Present case study provides an example of in-stent stenosis occurring 9 months after the placement of a Neuroform stent to support the coil embolization of an unruptured posterior communicating artery aneurysm.
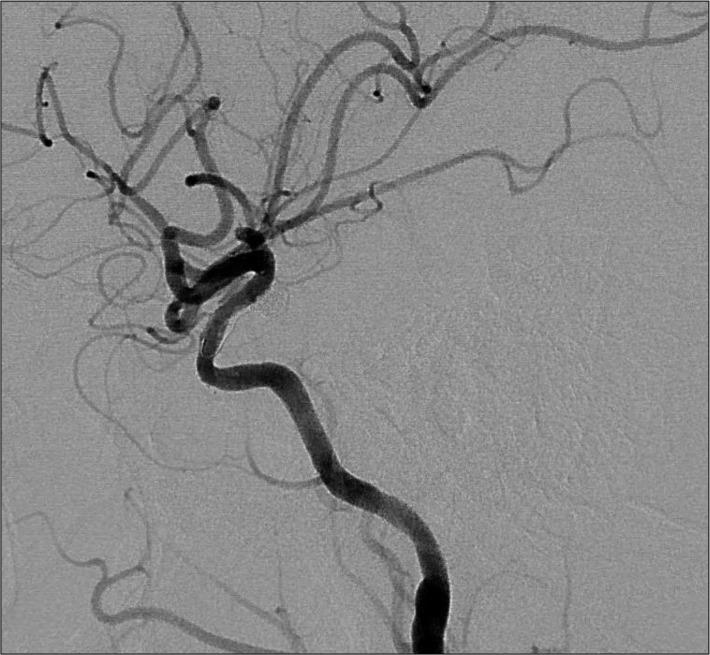
A 60-year-old female who had no significant prior medical history was refer to our clinic for management of an incidental left posterior communicating artery (PCOM) aneurysm that was initially diagnosed on the basis of magnetic resonance imaging findings. Digital subtraction angiography (DSA), performed under local anesthesia, confirmed a 4.3 mm sized aneurysm of the left PCOM with 3.8 mm neck (Fig. 1). After a discussion about management options-serial image follow-up, surgical clipping, or endovascular coil embolization-and associated risks, the patient elected to proceed with coil embolization. Because of unfavorable dome to neck ratio, a stent-assisted coil embolization was planned to cover the aneurysm neck and preserve the parent artery. The patient was pretreated for 5 days with 75 mg of clopidogrel daily and 100 mg of acetylsalicylic acid daily.
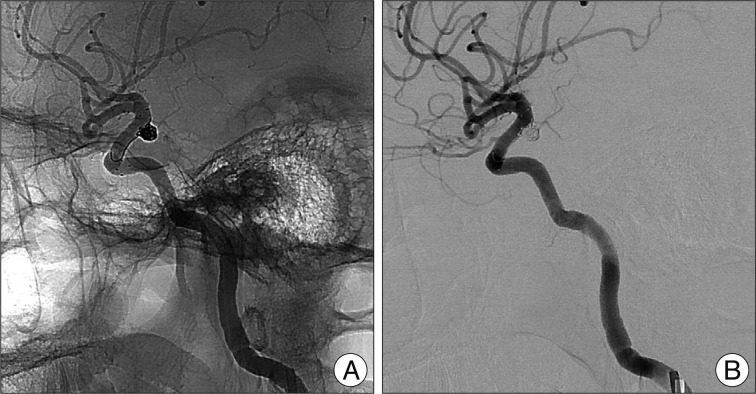
The patient was fully awake during the procedure, and electrocardiogram, arterial oxygen saturation, and blood pressure were appropriately monitored. Baseline activated clotting times (ACT) were obtained before the procedure. Percutaneous access was obtained via the right femoral artery, and a 6 Fr sheath was inserted. Systemic heparinization and a bolus injection of heparin (3000 IU) were administered before the therapeutic procedure; an additional 1000 IU bolus of heparin was administered every hour to maintain an ACT >250 seconds throughout the procedure. A 6 Fr guiding catheter (Envoy, Cordis, FL, USA) was navigated into the distal cervical internal carotid artery (ICA) segment, and pre-procedural angiograms were obtained in orthogonal planes. After establishing the optimal working angles for coil embolization, a 300-cm long, 0.014-inch Transcend 14 microwire (Boston Scientific, Natick, MA, USA) was navigated into the third segment of the left middle cerebral artery (M3). A 4.5×20 mm Neuroform stent delivery system was then advanced over the microwire and positioned across neck of the aneurysm by using roadmap image and stent markers, and the position was angiographically confirmed. A microcatheter (Echelon 10, eV3, USA) was then navigated with a Transcend 14 microwire through the guiding catheter into the aneurysm sac. After a stable microcatheter position within the aneurysm was attained, the Neuroform stent was deployed at the neck portion of aneurysm. The stent was easily and accurately deployed over the microwire. Coil embolization through the jailed microcatheter was then initiated. The aneurysm was completely occluded with employment of seven detachable coils. The final angiogram demonstrated complete occlusion of the aneurysm with final one coil loop extrusion between stent strut and vessel wall, the stent remained widely patent, and the stented segments of the ICA appeared smooth and without evidence of dissection or spasm (Fig. 2). The patient's post-operative period was uneventful, and she discharged on the third post-operative day. After the procedure, the patient was maintained on dual antiplatelet therapy for 9 months.
After 9 months, we routinely performed a follow-up DSA. Although still experiencing persistent headache, she indicated no new neurologic complaints, and her neurologic examination was normal at that time. A DSA confirmed diffuse stenosis throughout the stented segment of the ICA, most severely within the mid-portion of the stent. A stenosis rate was measured approximately more than 90% (Fig. 3). Collateral circulation was provided mainly by the right ICA through the anterior communicating artery.
In view of the severity of the stenosis while on dual antiplatlet therapy, angioplasty of the stenotic segment of the ICA was performed. A 2.75×9 mm-sized Gateway balloon (Boston Scientific, Natick, MA, USA) was used to dilate the stenotic ICA segment with slow inflations performed for about 10 seconds to a maximum pressure of 6 atmospheres. Postangioplasty control angiography demonstrated a marked improvement in the caliber of the lumen with improved distal flow throughout the ICA (Fig. 4). The patient remained asymptomatic on dual antiplatelet therapy and is awating further follow-up.
The introduction of intracranial stents has significantly contributed to the treatment options for coil occlusion of wide-necked or fusiform aneurysms. Stent-assisted coil embolization helps to prevent protrusion of the coils into the parent vessel12). As well as this mechanical effect, experimental studies have demonstrated that stents have hemodynamic and biologic effects1,9,11,14). In theory, flow diversion can cause blood to become stagnant and lead to progressive thrombosis and the promotion of endothelialization that can help the aneurysmal neck close7). Accordingly, these additional effects of stents may enhance the durability of coiled aneurysms. At the same time, neointimal proliferation occurring within the stented segment could conceivably result in a significant degree of parent vessel compromise3,6,8,15).
The Neuroform stent is the first stent designed specifically for use in the cerebrovasculature to support aneurysm treatment and has an open cell design, which gives it high navigability5,7). Despite these advantages, there is limited knowledge about long-term effects of the Neuroform stent on the cerebrovasculature. The rate of angiographic or clinically significant in-stent stenosis after Neuroform stent-assisted aneurysm treatment is currently not characterized, but is thought to be very low11).
In-stent stenosis is a common and well-described delayed complication of angioplasty and stenting for atheromatous disease. This process has been reported to occur in all vascular distributions after stenting. The deployment of a balloon-mounted stent inevitably causes endothelial injury over the treated vascular segment. There is a proliferation and activation of regional smooth muscle cells, resulting in neointimal hyperplasia, which can lead to restenosis within the stent10). Neointimal hyperplasia is histologically marked by proliferation of smooth muscle cell and immoderate production of extracellular matrix6,15). There is limited data documenting the rate of restenosis after the deployment of stents within the cerebrovasculature for atheromatous disease. In the stenting of symptomatic atherosclerotic lesions in the vertebral or intracranial arteries trial, greater than 50% delayed in-stent narrowing was observed in 32% of intracranial lesions and 43% of extracranial lesions treated with the balloon mounted Neurolink stent (Guidant Corp., Menlo Park, CA, USA)13).
Although neointimal hyperplasia and in-stent stenosis have been described to occur at variable rates after angioplasty or the deployment of a balloon-mounted stent for atheromatous disease within the cerebrovasculature and elsewhere, the available data would not be expected to translate directly to the Neuroform stent, which is typically deployed in nonstenotic nonatheromatous vessels. The self-expanding Neuroform stent is deployed without concurrent balloon angioplasty and has a very low intrinsic radial force. As such, the endotherial disruption injury resulting from Neuroform stent deployment is negligible in comparison with either angioplasty alone or the deployment of a conventional balloon-mounted stent. In 2006, Fiorella et al.6) presented the largest available case series describing the fate of Neuroform stents deployed within intracranial arteries. They reported that nine (5.8%) of 156 aneurysms treated with the Neuroform stent were observed to have moderate to severe (>50%) in-stent stenosis and estimated that delayed Neuroform in-stent stenosis, occurring in 5.8% of cases, was not a rare phenomenon. Symptomatic stenosis among them occurred in two cases (1.3%).
In the present case, noncompliant balloon angioplasty was performed, although the stenosis was asymptomatic, in view of the severity of the stenosis (stenosis rate >90%) while on dual antiplatlet therapy. The spontaneous resolution of in-stent stenosis was first described by Fiorella et al.6) and they observed that of the seven asymptomatic patients with a delayed Neuroform in-stent stenosis, four demonstrated some degree of spontaneous resolution at follow-up. They commented that in asymptomatic patients, a strategy of "watchful waiting" might be effective because many patients demonstrated partial or complete resolution at follow-up.
Antiplatelet resistance, which means insufficient inhibition of platelet aggregation by antiplatelet agents in vitro suggests another mechanism of in-stent stenosis of Neuroform stent. Reported data is highly variable, ranging from 3 to 85% for asprin and 28 to 44% for clipidogrel2). In our case, we did clopidogrel resistance test but she was a good responder. Considering the present case retrospectively, we made hasty decision about the treatment for in-sent stenosis and it is necessary to establish the treatment protocol for in-stent stenosis.
We presented a rare case of severe in-stent stenosis occurring as a delayed complication of Neuroform stent-assisted coil embolization of an unruptured intracranial aneurysm. Although in-stent stenosis was treated well with balloon angioplasty in the present case, the treatment protocol for in-stent stenosis should be established and a multi-disciplinary treatment approach is required.
References
1. Cantón G, Levy DI, Lasheras JC, Nelson PK. Flow changes caused by the sequential placement of stents across the neck of sidewall cerebral aneurysms. J Neurosurg. 2005; 103:891–902. PMID: 16304994.

2. Collet JP, Montalescot G. Platelet function testing and implications for clinical practice. J Cardiovasc Pharmacol Ther. 2009; 14:157–169. PMID: 19721130.

3. Fiorella D, Albuquerque FC, Deshmukh VR, McDougall CG. In-stent stenosis as a delayed complication of neuroform stent-supported coil embolization of an incidental carotid terminus aneurysm. AJNR Am J Neuroradiol. 2004; 25:1764–1767. PMID: 15569743.
4. Fiorella D, Albuquerque FC, Deshmukh VR, McDougall CG. Usefulness of the Neuroform stent for the treatment of cerebral aneurysms : results at initial (3-6-mo) follow-up. Neurosurgery. 2005; 56:1191–1201. discussion 1201-1202. PMID: 15918935.
5. Fiorella D, Albuquerque FC, Han P, McDougall CG. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery. 2004; 54:6–16. discussion 16-17. PMID: 14683536.

6. Fiorella D, Albuquerque FC, Woo H, Rasmussen PA, Masaryk TJ, McDougall CG. Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery. 2006; 59:34–42. discussion 34-42. PMID: 16823298.

7. Ismail Alhothi A, Qi T, Guo S, Shi Z, Liang F, Yang L, et al. Neuroform stent-assisted coil embolization : a new treatment strategy for complex intracranial aneurysms. Results of medium length follow-up. Neurol Neurochir Pol. 2010; 44:366–374. PMID: 20827610.

8. Kearney M, Pieczek A, Haley L, Losordo DW, Andres V, Schainfeld R, et al. Histopathology of in-stent restenosis in patients with peripheral artery disease. Circulation. 1997; 95:1998–2002. PMID: 9133506.

9. Kim M, Levy EI, Meng H, Hopkins LN. Quantification of hemodynamic changes induced by virtual placement of multiple stents across a wide-necked basilar trunk aneurysm. Neurosurgery. 2007; 61:1305–1312. discussion 1312-1313. PMID: 18162911.

10. Kipshidze N, Dangas G, Tsapenko M, Moses J, Leon MB, Kutryk M, et al. Role of the endothelium in modulating neointimal formation : vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 2004; 44:733–739. PMID: 15312851.

11. Lopes D, Sani S. Histological postmortem study of an internal carotid artery aneurysm treated with the Neuroform stent. Neurosurgery. 2005; 56:E416. discussion E416. PMID: 15670395.

12. Pride GL Jr, Horowitz MB, Purdy PD. Endovascular problem solving with intravascular stents. AJNR Am J Neuroradiol. 2000; 21:532–540. PMID: 10730647.
13. SSYLVIA Study investigators. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA) : study results. Stroke. 2004; 35:1388–1392. PMID: 15105508.
14. Wanke I, Forsting M. Stents for intracranial wide-necked aneurysms : more than mechanical protection. Neuroradiology. 2008; 50:991–998. PMID: 18807024.

15. Yoon KW, Kim YJ. In-stent stenosis of stent assisted endovascular treatment on intracranial complex aneurysms. J Korean Neurosurg Soc. 2010; 48:485–489. PMID: 21430973.

Fig. 1
Left internal carotid artery angiogram depicting a broad-based aneurysm arising from the posterior wall of the left internal carotid artery.

Fig. 2
Unsubtracted image (A) depicting a Neuroform stent that was indicated by four radiopaque stent markers positioned across the neck of the coiled aneurysm with final one coil loop extrusion between stent cell and vessel wall. Subtracted image from a left internal carotid artery angiogram (B) demonstrating complete occlusion of the aneurysm. The stent is widely patent and the stented segment of the parent vessel appears completely intact. Specifically, there is no evidence of dissection or vasospasm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download