INTRODUCTION
Meralgia paresthetica (MP) is a mononeuropathy resulting from the compression of the lateral femoral cutaneous nerve (LFCN) as it crosses between the anterior superior iliac spine (ASIS) and the inguinal ligament to enter the thigh
20,
28). The nerve receives sensory input from the skin of the anterolateral thigh (
Fig. 1A). Affected patients consequently experience a very prominent painful dysesthesia and, less commonly, vasomotor disturbance in the cutaneous distribution of the nerve. The term MP was first coined by Roth
21) in 1895. However, despite its long-term recognition, very few studies have been published on the surgical management and outcome of this condition
10,
17,
18,
23,
26).
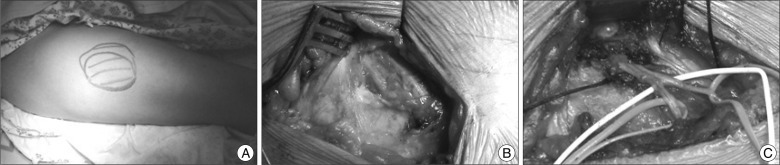 | Fig. 1Operative findings of decompression with neurolysis. A : A clinical photo showing the maximal painful dysesthetic area of right lateral theigh. B : Initial exposure of the course of lateral femoral cutaneous nerve. Note two branches, anterior and posterior branches of lateral femoral cutaneous nerve. C : Final operative photograph showing complete decompression along the course of lateral femoral cutaneous nerve. Decompression of the nerve was performed at the level of the iliac fascia, the inguinal ligament, and the fascia of the thigh distally. 
|
Two types of operative techniques, decompression with neurolysis and transection of LFCN, have been proposed to be effective for MP
23,
27). Transection of the nerve at its exit from the pelvis is a simple and effective procedure provided the nerve can be found
,
26,
28). However, such a procedure results in anesthesia over the lateral thigh
23). Symptomatic relief expected of neurolysis has not been consistently reported in the literature
3,
14,
17,
18,
23). In an effort to provide clarification, we report long-term results of neurolysis for MP from our collected series of 11 cases.
Go to :

DISCUSSION
The syndrome involving a burning, tingling, and numb sensation in the anterolateral area of the thigh, with variable reduction of sensation in the distribution of the lateral femoral cutaneous nerve, was first described by Bernhardt
4) in 1895 and named meralgia paresthetica (MP; derived from the Greek word meros meaning thigh and algos, meaning pain) by Roth
20) in the same year. MP has at least 80 etiologies and can be categorized as spontaneous or iatrogenic
1,
5,
7,
9,
12,
13). Spontaneous causes include mechanical factors such as obesity, pregnancy, and other conditions associated with increased intraabdominal pressure
28). The wearing of belts, corsets, and tight trousers can also result in direct pressure on the LFCN
6), and pelvic benign masses such as uterine fibrinoids as presenting as MP have been reported
24,
25). Other causes of spontaneous MP include radiologically degenerative pubis symphysis, limb length discrepancy, and osteoid sarcoma in more than one-third of pediatric patients following treatment
13).
In the classic form of MP, the symptoms are fairly stereotypical and the diagnosis should be fairly straightforward for the astute clinician
9,
19). However, diagnosis of MP is not always straightforward in clinical practice
19). This condition can be confused with, and in a small group of patients it can coexit with, lumbosacral radicular pain
11,
19,
23). In our cases, the diagnosis of MP was fairly delayed and was not diagnosed until interviews conducted in our outpatient clinic. We speculate that this delay in diagnosis of MP in our country may have been related to poor understanding of this entity by some of the attending primary physicians and orthopedic and neurosurgical professionals. When there is doubt about the diagnosis following history-taking and physical examination, electrodiagnostic testing can be used. Side-to-side amplitude difference of the SNAP of the LFCN is a more sensitive predictor of MP than the absolute amplitude of the SNAP. In fact, a side-to-side amplitude ratio exceeding 2.3 combined with a SNAP <3 microvolts yields a specificity >of 98 %
21).
In patients where a clinical diagnosis of MP has been made, a diagnostic block may be made with 8 mL of 0.25% bupivacaine
7,
22). All patients who underwent neurolysis in our series responded temporarily with repeated blocks of LFCN, and we performed differential blockade of LFCN at least three times before making a decision to decompress the LFCN.
Surgical techniques for MP include neurolysis of the constricting tissue, neurolysis and transposition of the LFCN, and transection with excision of a portion of the LFCN. Each has its own advantage and disadvantages. Neurolysis is a physiologic procedure that seeks to preserve the integrity of the LFCN. However, reported results of neurolysis vary from 60% to 95% due to presence of neuroma and anatomical variations in the course of the LFCN
3,
16,
17,
23). Only two reports have described neurolysis combined with transposition
2,
14) and this approach has not been tested in a clinical trial
12). Transection is another effective means to produce good results
28). However, sectioning of the LFCN can produce a permanent anesthetic area in the anterolateral thigh and there is a risk of neuroma formation.
Regarding neurolysis for MP, conflicting results with failure rates as high as 40% and success rates of 90% to 95% have been reported
3,
17,
18,
23,
27). Why surgery works for entrapment of other peripheral nerves such as the median and ulnar nerve, but may not work for LFCN is unresolving and intriguing. This dichotomy may be due to the difficulty in establishing a correct diagnosis of MP
17), inability to locate LFCN due to its anatomical variability
3), and inadequate decompression
2). In this regard, several measures have been advocated
23). Because the presentation of MP can be confused with other conditions, such as facet joint syndrome and trochanteric bursitis, electrodiagnostic studies and diagnostic block of LFCN should be performed for confirmation of diagnosis. The high variability of the location of LFCN needs to be particularly borne in mind during nerve exploration. Complete neurolysis of LFCN should be ensured with proximal and distal extension of decompressive maneuvers. In particular, the iliac fascia is to be divided when appropriate tension bands are often noted in compressing the nerve in its pelvic course. In addition, a sling of fascia posterior to the nerve in the region of inguinal ligament has been sought
23).
Siu and Chadran
23) reported the biggest series of neurolysis (45 cases) in MP surgery. In their 45 neurolysis procedures, 42 cases with follow-up of 4.1 years displayed complete and partial symptom improvements were noted in 33 (73%) and nine (20%) cases, respectively. No recurrence was noted. Analysis of clinical variables demonstrated that the duration of symptoms preoperatively did not affect the rate of complete symptom relief, but obese patients (body mass index >30) was six-times more likely to have incomplete surgery at the long-term follow-up.
The presence of a neuroma and the frequency of anatomical variation in the course of LFCN may render neurolysis difficult. Therefore, transection of the LFCN is another effective means of treating refractory MP. Williams and Trzil
28) reported excellent relief in 23 of 24 cases of transection. They stressed that sectioning of the LFCN offers uniformly good results and should be easily reproducible once adequate identification of the nerve is accomplished, and patients are willing to accept permanent anesthesia on an area of the anterolateral thigh in exchange for relief of their symptoms. Ivins
14), in another series of 14 adult patients with a follow-up over 3-6 years, reported the effectiveness of transection in case of recurrence of MP after initial relief by neurolysis. With these favorable results of transection, they advocated primary transection in adult patients experiencing symptoms for more than 1 year and patients who have persistent or recurrent symptoms. van Eerton et al.
26) evaluated transection and neurolysis in 21 refractory MP patients and found that transection (complete relief of symptoms in nine of 12 patients) was superior to neurolysis (complete relief of symptoms in three of 10 patients).
In our series, neurolysis was effective in relieving medically refractory MP. Neurolysis provided complete relief in nine patients (81.8%) and partial relief (18.2%) in two patients. There was no failure and recurrence in our series. Although previous authors stressed the relationship between obesity and recurrence of symptom, we could not find any correlation. With limited numbers of our series we cannot explain this finding. Since most papers dealing with surgeries for MP involved studies with Caucasians, a racial difference might be the reason. In a Korean report dealing with the surgical result of MP
16), the authors investigated the effectiveness of surgery in nine cases of neurolysis and two cases of transaction in iatrogenic MP caused by iliac bone graft harvesting for spinal surgery. Even in iatrogenic MP, transection and neurolysis was effective in relieving painful symptoms of MP in 10 out of 11 patients
16). Among the nine cases of neurolysis, complete relief was achieved in three patients, and partial relief was accomplished in four patients. Our results with neurolysis seems superior to this report, however, there was a difference in the characteristics of the enrolled patients. Our study deals MP patients with spontaneous cause, whereas the earliest study
15) dealt with iatrogenic MP following surgery. However, even in iatrogenic MP following surgery, it seems that neurolysis could bring adequate relief of symptoms of MP.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download