Abstract
Objective
The purpose of this study was to verify the appropriateness of ovariectomized rats as the osteoporosis animal model.
Methods
Twelve female Sprague-Dawley rats underwent a sham operation (the sham group) or bilateral ovariectomy [the ovariectomy (OVX) group]. Eight weeks after operations, serum biochemical markers of bone turnover were analyzed; osteocalcin and alkaline phosphatase, which are sensitive biochemical markers of bone formation, and C-terminal telopeptide fragment of type I collagen C-terminus (CTX), which is a sensitive biochemical marker of bone resorption. Bone histomorphometric parameters and microarchitectural properties of 4th lumbar vertebrae were determined by micro-computed tomographic (CT) scan.
Results
The OVX group showed on average 75.4% higher osteocalcin and 72.5% higher CTX levels than the sham group, indicating increased bone turnover. Micro-CT analysis showed significantly lower bone mineral density (BMD) (p=0.005) and cortical BMD (p=0.021) in the OVX group. Furthermore, the OVX group was found to have a significantly lower trabecular bone volume fraction (p=0.002).
Osteoporosis is a metabolic bone disease characterized by loss of bone mass and disruption of bone microarchitecture, and consequently increases the risk of fracture3,5,11,14). It is well known that estrogen plays a fundamental role in skeletal growth and bone homeostasis1-3,6). This is of particular importance in postmenopausal women, whose estrogen levels are naturally lowered, and in whom the incidence of osteoporosis is high, because a rapid decrease in estrogens in this population is the predominant cause of the imbalance between bone formation and bone resorption12).
Although no single animal model precisely mimics the human condition of osteoporosis, appropriate animal models can provide information on bone quality and structure not obtainable during clinical trials. Ovariectomy (OVX) provides the most popular model for studying events associated with postmenopausal osteoporosis with estrogen deficiency3,5,12,16), and it has been well established that ovariectomy elicits bone loss and increased bone turnover in rats3,5,7-9,11,12,16). Thus, OVX rat models of osteoporosis can mimic conditions in postmenopausal women, and are suitable for the evaluations of potential therapeutics designed to prevent or treat osteoporosis7-9,16).
In the present study, in an effort to devise a reliable and reproducible OVX rat model of osteoporosis, we performed histomorphometric analyses of the lumbar spine using micro-computed tomography (CT) scans and evaluated changes in serum bone turnover markers after ovariectomy in rats.
All animal experiments were performed in accordance with the animal care guidelines issued by the National Institute Health, and were approved by the Institutional Animal Care Committee at Kyungpook National University.
Twelve female Sprague-Dawley rats (11 weeks old, body weight 190-210 g) were purchased from Samtako Bio Inc. (Osan, Korea) and acclimated to conditions for one week before the experiment. Animals were housed in an air-conditioned room (relative humidity 45-65%) under a 12-h light/dark cycle at 22±2℃ and given free access to food and tap water. Acclimated rats were assigned to one of two groups, either a sham-operated group (Sham, controls; n=6) or a surgically ovariectomized group (OVX; n=6). At surgery, rats were initially anesthetized intraperitoneally with a mixture of xylazine (10 mg/kg) and ketamine (60 mg/kg). A 2 cm skin incision was then made just medial to the most bulging part of the back. After pulling away periovarian fat with the ovaries, complete bilateral ovariectomy was performed, and then muscle, fascia, and skin were sutured using 4-0 silk. The sham controls underwent the same surgical procedure, but ovariectomy was not performed.
Body weights were checked once a week throughout the 8-week experiment period. Eight weeks after surgery, all animals were sacrificed and blood samples were collected by cardiac puncture for serum isolation. Serum was separated by centrifugation (at 1500×g) and then stored at -80℃ until required for bone metabolic marker assays. Serum estrogen (estradiol, E2) was determined using an estradiol ELISA kit (ELISA, DRG instruments GmbH, Germany). In addition, 4th lumbar vertebrae were removed, fixed in a 3.7% formaldehyde in phosphate-buffered saline solution (pH 7.4) for 16 h and then stored (4℃) in 80% ethanol for bone mass measurements.
Serum osteocalcin levels and alkaline phosphatase (ALP) activities (both sensitive biochemical markers of bone formation) were determined using osteocalcin EIA kits (Nordic Bioscience Diagnostics, Herlev, Denmark) and QuantiChrome ALP assay kits (DALP-250, BioAssay Systems, CA, USA), respectively. Serum levels of C-terminal telopeptide fragment of type I collagen C-terminus (CTX), which is generated by the osteoclast and is a marker of bone resorption, were determined using RatLaps ELISA kits (Nordic Bioscience Diagnostics, Herlev, Denmark)3,6).
Bone histomorphometric parameters and the microarchitectural properties of 4th lumbar vertebrae were determined using a micro-CT system (eXplore Locus SP, GE Healthcare) with X-ray energy settings of 80 kV and 80 µA. Samples were scanned over one entire 3600 rotation at an exposure time of 3000 ms/frame. An isotopic resolution of 15-40 µm voxel size that displayed the microstructure of rat lumbar vertebra was selected, and the angle of increment around the sample was set to 0.4°, which resulted in the acquisition of 900 2D images. A modified Feldkamp cone-beam algorithm was used for 3D reconstruction.
For bone analysis, entire 4th lumbar vertebrae were selected as the region of interest. Image information was obtained based on the automatic domain values produced by the computer. Bone mineral contents (BMC), bone mineral densities (BMD), trabecular bone volume fractions (BV/TV, %), trabecular thicknesses (Tb.Th.), trabecular numbers (Tb.N.), trabecular separations (Tb.Sp.), and cortical bone mineral densities (Cr.BMD) were used for the quantitative analysis, which was performed using 2.0+ ABA Microview software provided with the micro-CT system13,14,17).
Mechanical spinal strength was determined by a three-point bending test. Each bone was positioned on the two lower supports of the anvil of a Universal Testing Machine (Instron 4202; Instron, Canton, MA, USA). Load was applied to the midportion of the 4th lumbar vertebrae using a crosshead speed of 1.5 mm/min for all the tests. The load versus displacement data were recorded automatically by the Instron software (INSTRON series IX Automated Materials Tester, version 8.04.00), which subsequently calculates the mechanical parameters from the load-displacement curves.
All statistical comparisons were made using SPSS 17.0. Data are expressed as means+standard deviations. Repeated measure ANOVA was used to compare body weights in the OVX and sham groups. The two-sample t test was used to identify significant differences between the groups, and p values of <0.05 were considered significant.
Body weights were checked once a week throughout the 8-week experiment period. Fig. 1 shows changes in mean body weights in the two groups with different time. At the start of the experiment, body weights were similar in the two groups (p=0.368). However, at 4 weeks after surgery, mean body weight was significantly greater in the OVX group compared with the sham group, and this significance was maintained throughout the period (p=0.015, 0.037, and 0.011 at 4, 6, and 8 weeks, respectively).
As shown in Fig. 2, 8 weeks after surgery, serum estradiol levels were significantly lower in the OVX group (1.61±0.62 pg/mL vs. 19.95±3.47 pg/mL; p=0.001).
Fig. 3A, B summarize the serum levels of the biochemical markers
of bone formation, osteocalcin, and ALP, and Fig. 3C shows CTX serum levels, which are the sensitive markers of bone resorption. The OVX group had on average a 75.4% higher osteocalcin level than the sham group, which was statistically significant (p=0.002). Although serum ALP levels were 83.66±21.24 IU/L in the sham group and 142.72±58.72 IU/L in the OVX group, they were not significantly different (p=0.136). On the other hand, serum CTX levels in the OVX group were 72.5% higher than in the sham group, which was significant (p=0.018).
As shown in the representative micro-CT images, OVX rats had fewer trabecular bone structures than the sham controls (Fig. 4).
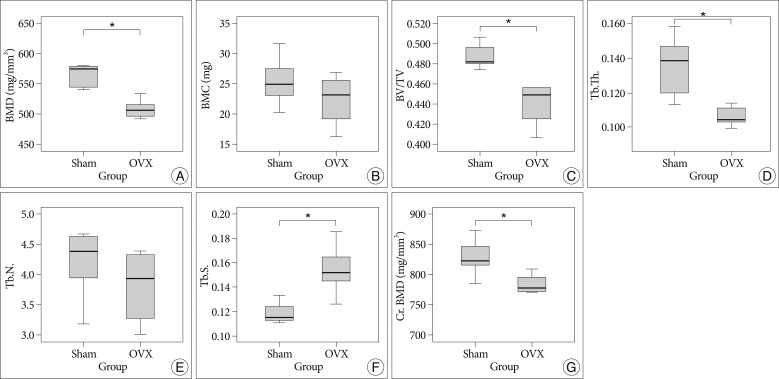
In the analyses of micro-CT scans of 4th lumbar vertebrae, the OVX group showed a significant 12% reduction in BMD, and a significant 9.1% reduction in trabecular bone volume fraction (BV/TV) (p=0.005 and p=0.002, respectively). Furthermore, mean BMC in the OVX group was lower than in the sham group (22.38±4.12 mg vs. 25.52±4.37 mg, respectively), though this was not significantly different (p=0.252). In addition, OVX rats showed a significant decrease in Tb.Th. (p=0.011) and a significant increase in Tb.Sp. (p=0.006), when compared to the sham group. However, Tb.N. in the OVX group were lower than that of the sham group (4.2±0.6 in the sham group and 3.8±0.5 in the OVX group), which was not significantly different (p=0.351).
In the histomorphometric analysis of cortical bone, the OVX group also showed a significant 6.2% reduction in Cr.BMD versus the sham group (p=0.021) (Fig. 5).
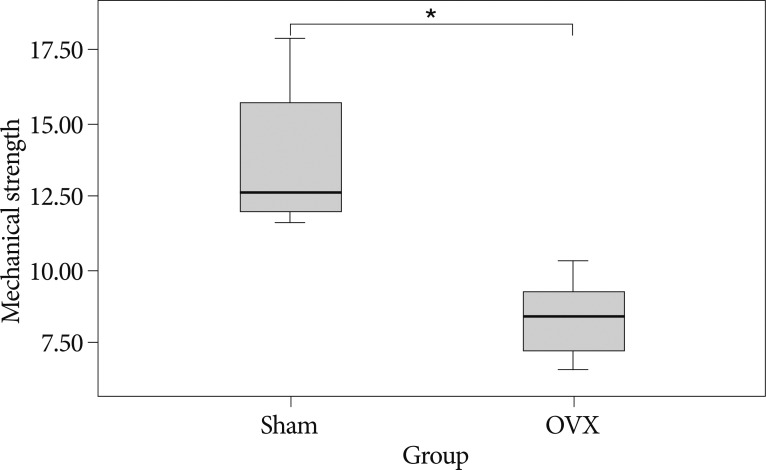
The three point bending test showed that the 4th lumbar vertebrae of OVX group have on average 39% lower maximal load value compared to that of the sham group (p=0.001) (Fig. 6).
The incidence of osteoporosis is increasing rapidly, especially in the elderly population. Osteoporosis is divided into two types : postmenopausal and senile osteoporosis. The causes of postmenopausal osteoporosis are accelerated bone resorption and systemic calcium imbalance due to menopause-induced estrogen deficiency3,5,11,12,14).
The animal models of osteoporosis are essential for anticipating treatment efficacy and safety2,10), and of the various animal models used, rodents have numerous advantages, for example, they are inexpensive, easy to house, the rapid generation time, and their skeletons are sensitive to the loss of ovarian hormones18). Ovariectomized models are most frequently used for studying postmenopausal osteoporosis with estrogen deficiency. There is extensive literature studying OVX rat including the histomorphometric changes and the biochemical markers of bone turnover2-6,10,11,13,14,17).
In the present study, we investigated changes in bone turnover and bone loss after ovariectomy in Spraque-Dawley rats. At 8 weeks after ovariectomy, serum estradiol levels in the OVX group were significantly lower than in the sham group (1.64±0.62 pg/mL vs. 19.94±3.46 pg/mL), which supports the presence of ovarian deficiency in the OVX model. Furthermore, as was expected, mean body weights were greater in the OVX group than in the sham group (p=0.011). Omi et al.9) described that weight gain efficiency (body weight gain/food intake) in their OVX group was significantly greater than in the sham group. Turner et al.18) also found that ovariectomy increases body weight more in OVX group than in sham group. Accordingly, our results are comparable to those of previous studies that have used an OVX rat model3,7-9,12,16,18).
Biochemical markers of bone turnover have been widely used as measures of the status of bone remodeling. The bone remodeling cycle involves the achievement of balance between the bone resorbing activities of osteoclasts and the bone forming activities of osteoblasts. After menopause, bone resorption markers increase due to the activation of bone resorption by estrogen deficiency, and bone formation increases to fill the higher number of resorption cavities, and as a result, increase in their serum levels. The currently accepted mechanism of skeletal bone loss in estrogen deficient rats is that of an imbalance in bone turnover, that is, where bone resorption exceeds bone formation1,16). The biochemical markers of bone turnover used in clinical and experimental studies include osteocalcin and ALP (a sensitive marker of bone formation) and CTX (a sensitive marker of bone resorption).
In a study of ovariectomized rats, Kim et al.3) found higher plasma osteocalcin and CTX concentrations in their OVX group than in sham operated animals, and similarly, it has been reported that estrogen deficiency increases serum ALP, osteocalcin, and CTX levels, which indicated the increased bone turnover2,9,12,15). In the present study, ovariectomized animals were found to have higher osteocalcin, ALP and CTX level than sham controls, indicating increased bone turnover due to menopause-induced estrogen deficiency, which is entirely consistent with previous studies2,9,12,15).
In ovariectomized animals, as in postmenopausal women, bone loss induced by ovarian deficiency mainly results from trabecular bone loss3). Although BMD measurements could be performed by dual-energy X-ray absorptiometry, micro-CT can also be used for monitoring bone microarchitecture and BMD in small animals. Scanned micro-CT images can separate cancellous and cortical bone, and therefore, they can be used to produce realistic depictions of apparent and tissue BMDs, which in turn reflect degrees of bone mineralization. In the present study, we analyzed bone histomorphometric parameters and microarchitectural properties, BMD, BV/TV, %, Tb.Th., Tb.N., Tb.Sp., and Cr.BMD using a micro-CT system.
Many studies have demonstrated bone loss induced by estrogen deficiency in ovariectomized rats. Lei et al.5) reported a significant decrease in femoral BMD in 8-week OVX rats as compared 8-week sham rats. In a recent study of histological observa-tions of bone resections of vertebrae and femurs, bone mass was found to be obviously reduced in ovariectomized rats than in sham group10). Similarly, in the present study, we also observed that BMD and BV/TV in 4th lumbar vertebrae and cortical BMD were significantly lower in the OVX group. These findings support the notion that our model successfully mimics the effects of osteoporosis.
When considering animal models of osteoporosis, it is important that bone sites should be taken into account. It is well known that, in ovariectomized animals, cortical bone loss does not match that of trabecular bone2,9). In a study of ovariectomized rats, Ferretti et al.2) reported that bone mass started to decrease earlier and more extensively in trabecular than in cortical bone. We observed similar findings in the present study. In particular, mean BMD of 4th lumbar vertebra at 8 weeks after surgery, had reduced by 12.0%, but mean cortical BMD had only reduced by 6.2%.
The present study demonstrates that ovariectomy in rats significantly increases bone turnover, and this was confirmed by elevated levels of bone turnover markers. Furthermore, it shows a significant loss of trabecular bone in lumbar vertebrae 8 weeks after ovariectomy. Finally, our study demonstrates that the ovariectomized rat model offers a reproducible and reliable model of osteoporosis.
References
1. Canpolat S, Tug N, Seyran AD, Kumru S, Yilmaz B. Effects of raloxifene and estradiol on bone turnover parameters in intact and ovariectomized rats. J Physiol Biochem. 2010; 66:23–28. PMID: 20428990.

2. Ferretti M, Bertoni L, Cavani F, Zavatti M, Resca E, Carnevale G, et al. Influence of ferutinin on bone metabolism in ovariectomized rats. II: role in recovering osteoporosis. J Anat. 2010; 217:48–56. PMID: 20492429.

3. Kim TH, Jung JW, Ha BG, Hong JM, Park EK, Kim HJ, et al. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J Nutr Biochem. 2011; 22:8–15. PMID: 20233653.

4. Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int. 1985; 37:594–597. PMID: 3937580.

5. Lei Z, Xiaoying Z, Xingguo L. Ovariectomy-associated changes in bone mineral density and bone marrow haematopoiesis in rats. Int J Exp Pathol. 2009; 90:512–519. PMID: 19765105.

6. Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, Nasti A, et al. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004; 145:3115–3121. PMID: 15059958.

7. Miller SC, Bowman BM, Jee WS. Available animal models of osteopenia--small and large. Bone. 1995; 17:117S–123S. PMID: 8579907.
8. Mosekilde L. Assessing bone quality--animal models in preclinical osteoporosis research. Bone. 1995; 17:343S–352S. PMID: 8579937.
9. Omi N, Ezawa I. The effect of ovariectomy on bone metabolism in rats. Bone. 1995; 17:163S–168S. PMID: 8579912.

10. Palumbo C, Ferretti M, Bertoni L, Cavani F, Resca E, Casolari B, et al. Influence of ferutinin on bone metabolism in ovariectomized rats. I : role in preventing osteoporosis. J Bone Miner Metab. 2009; 27:538–545. PMID: 19333679.

11. Park SB, Lee YJ, Chung CK. Bone mineral density changes after ovariectomy in rats as an osteopenic model : stepwise description of double dorso-lateral approach. J Korean Neurosurg Soc. 2010; 48:309–312. PMID: 21113356.

12. Riggs BL, Khosla S, Melton LJ 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002; 23:279–302. PMID: 12050121.

13. Sheng ZF, Dai RC, Wu XP, Fang LN, Fan HJ, Liao EY. Regionally specific compensation for bone loss in the tibial trabeculae of estrogen-deficient rats. Acta Radiol. 2007; 48:531–539. PMID: 17520429.

14. Shiraishi A, Miyabe S, Nakano T, Umakoshi Y, Ito M, Mihara M. The combination therapy with alfacalcidol and risedronate improves the mechanical property in lumbar spine by affecting the material properties in an ovariectomized rat model of osteoporosis. BMC Musculoskelet Disord. 2009; 10:66. PMID: 19527501.

15. Szulc P, Delmas PD. Biochemical markers of bone turnover : potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int. 2008; 19:1683–1704. PMID: 18629570.

16. Thompson DD, Simmons HA, Pirie CM, Ke HZ. FDA Guidelines and animal models for osteoporosis. Bone. 1995; 17:125S–133S. PMID: 8579908.

17. Tommasini SM, Morgan TG, van der Meulen MCh, Jepsen KJ. Genetic variation in structure-function relationships for the inbred mouse lumbar vertebral body. J Bone Miner Res. 2005; 20:817–827. PMID: 15824855.

18. Turner AS. Animal models of osteoporosis--necessity and limitations. Eur Cell Mater. 2001; 1:66–81. PMID: 14562261.
Fig. 1
Graph showing temporal changes of body weights in the two study groups. Values represent mean+standard deviations (n=12). *p<0.05 for OVX vs. sham group comparisons. OVX : ovariectomy.
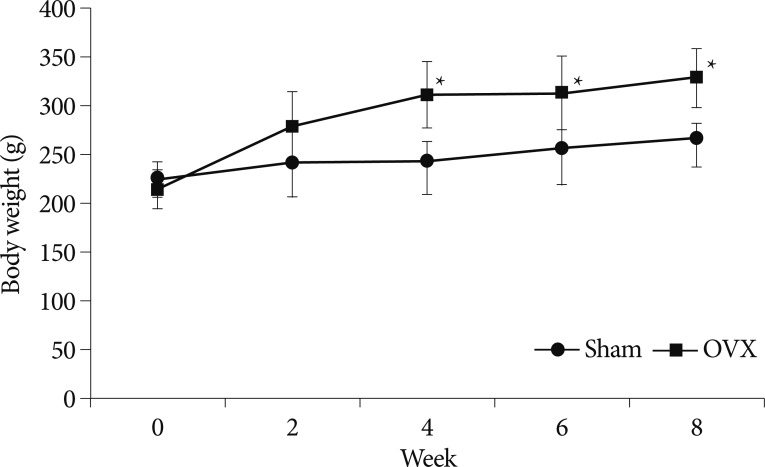
Fig. 2
Serum estrogen levels in the two study groups at 8 weeks after surgery. *p<0.05 for the OVX group compared to the sham group. OVX : ovariectomy.
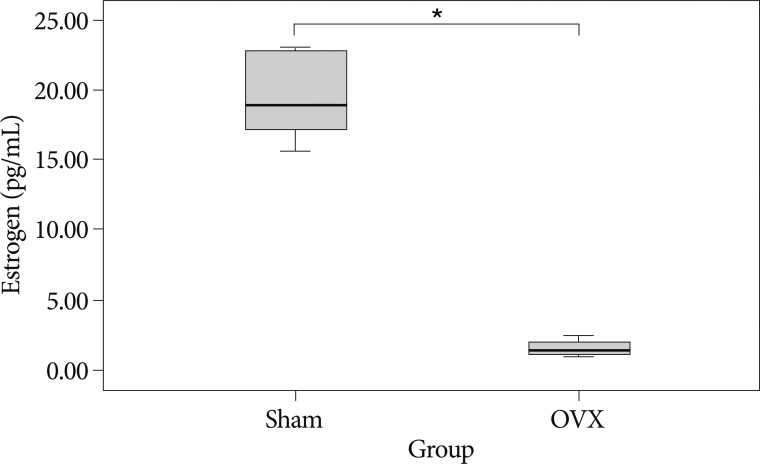
Fig. 3
The serum osteocalcin (A) and ALP (B) concentrations were used as markers of bone formation. Type I collagen CTX (C) concentration was used as a marker of bone resorption. Values are means+standard deviations (n=12). *p<0.05 for the OVX vs. sham group comparisons. ALP : alkaline phosphatase, CTX : C-terminus, OVX : ovariectomy.

Fig. 4
Representative micro-CT images of the two groups; the sham group (A) and the OVX group (B). OVX rats had less trabecular bone than sham controls at 8 weeks after surgery. OVX : ovariectomy.
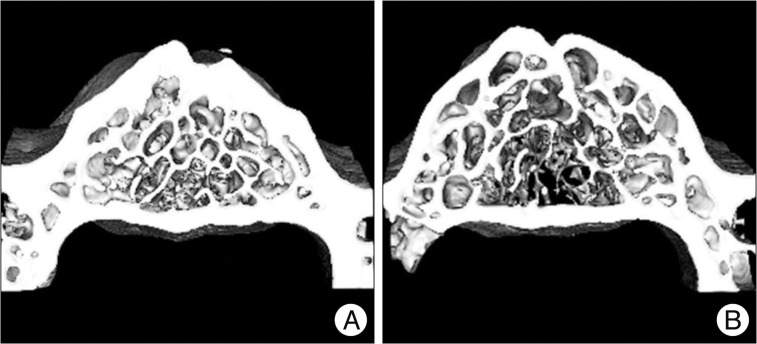
Fig. 5
Bar graph showing the result of histomorphometric analyses of 4th lumbar vertebrae in the sham and ovariectomy (OVX) groups; Bone mineral density (BMD) (A), bone mineral content (BMC) (B), trabecular bone volume fraction (BV/TV) (C), trabecular thickness (Tb.Th.) (D), trabecular number (Tb.N.) (E), trabecular separation (Tb.Sp.) (F), cortical bone mineral density (Cr.BMD) (G). *p<0.05 for OVX vs. sham group comparisons.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download