Abstract
Objective
This retrospective study was performed to evaluate the role of chemotherapy in the management of patients with anaplastic astrocytoma (AA).
Methods
We compared the survival outcome among the 3 different treatment protocol groups in a single institution. A total of 86 patients (39 men and 47 women) with newly diagnosed AA after surgery were analyzed. Among them, 31 patients (36.0%) were treated with radiotherapy only (RT Group), 30 patients (34.9%) were treated with nimustine-cisplatin chemotherapy before RT (ACNU-CDDP group), and 25 patients (29.1%) were treated with procarbazine, lomustine and vincristine (PCV) chemotherapy after radiotherapy (PCV group).
Results
The median survival was 14.0, 30.0 and 72.0 months in RT, ACNU-CDDP, and PCV group, respectively and showed significant differences (RT vs. ACNU-CDDP; p=0.039, RT vs. PCV; 0.002, ACNU-CDDP vs. PCV; 0.045). PCV group showed less toxicity rate (5 patients; 20%) than ACNU-CDDP group (12 patients; 40%), while only 3 patients (9.6%) in RT group experienced grade 3 or 4 toxicities.
The survival of patients with malignant glioma remains poor, with a median survival of 2 to 3 years for patients with anaplastic astrocytoma (AA) albeit the advancement of treatments including surgical resection, radiation therapy, and chemotherapy3). Also, the standard treatment for AA has been stagnant for decades with surgery followed by radiotherapy only1,14). The role of chemotherapy for AA is not fully established although the possibilities of benefit for prolonging of survival had been proposed2,13-15). Establishment of standard management protocols for AA is even more difficult due to its low incidence which is the major obstacle in designing the large-scale randomized prospective study. Many previous studies for the management of malignant glioma pooled both grades III or IV astrocytic tumors, and need for separate study for AA was advocated after sub-analysis of the study population6,8,10). In this study, we analyzed a relatively large series of 86 newly diagnosed AA patients to evaluate the role of chemotherapy for survival outcome.
A total of 86 newly diagnosed AA patients between 1990 and 2004, were included in this single-center retrospective study. All patients were histologically proved to be AA according to the WHO 2007 classification after surgical resection in 47 and stereotactic biopsy in 399). The median age was 40.9 years (range, 18-70 years), 39 patients were male and 47 patients were female. Preoperative brain magnetic resonance images revealed that 47 patients had enhancing lesion while 39 patients had no focal lesion of enhancement. Performance status after surgery expressed by Karnofsky performance score (KPS) of a whole group was good enough to be same of more than 70 in 82 patients (95.4%).
Three different treatment protocols were applied to patients with AA enrolled in this study. Among them, 31 patients (36.0%) were treated with radiotherapy only (RT Group). The complete scheduled radiation dose of 61.2 Gy was delivered and no other treatment was added to this group. Another group of 30 patients (34.9%) were treated with nimustine-cisplatin chemotherapy before RT (ACNU-CDDP group). This treatment was scheduled to receive 2 cycles of ACNU-CDDP neoadjuvant chemotherapy, followed by conventional radiotherapy. The neoadjuvant chemotherapy with ACNU (40 mg/m2/day) and CDDP (40 mg/m2/day) was administered by continuous infusion for 72 h and was repeated after 6 weeks. Among 30 patients, 20 patients completed 2 cycles of ACNU-CDDP while 10 patients received only 1 cycle and proceeded to radiotherapy because of toxicity. The other 25 patients (29.1%) were treated with PCV (procarbazine, lomustine and vincristine) chemotherapy after radiotherapy (PCV group). PCV chemotherapy started within 4 weeks after the end of radiotherapy. Each cycle consisted of lomustine 110 mg/m2 orally on day 1, procarbazine 60 mg/m2 orally on days 8 to 21, and vincristine 1.4 mg/m2 intravenously on days 8 and 29. Cycles were to be repeated every 6 weeks. Due to the intolerance, only 5 patients completed all 12 cycles while 8 patients received 6 to 11 cycles and 12 patients were treated with less than 6 cycles of PCV. In the RT group, their median age was 42 and 16 patients (52%) underwent resection of the mass including gross total removal, near total removal, subtotal removal, and partial removal. The other 15 patients underwent biopsy only to confirm pathologically. Median age of PCV group was 42 and 9 patients underwent only biopsy. The median age for ACNU-CDDP group was 40.5. Among them, 15 patients underwent biopsy. In three groups, the most of patients had above 70 KPS score. Table 1 summarizes the baseline characteristics of each group and shows that they are well-balanced.
Salvage treatments were performed to each groups during clinical observation period. Various protocols of salvage chemotherapy including PCV, ACNU-CDDP, amd temozolomide (TMZ) were tried. The chemotherapy was chosen according to the physicians' decision and clinical patients' status. Toxic effects were graded in accordance with the NCI CTCAE, version 3.0. Statistical analysis included ANOVA and chi-square test for parametric comparisons. All statistical significance was accepted at probability values of less than 0.05. The Kaplan-Meyer method was used to estimate the overall survival distributions. The log-rank test was used to test the differences in the overall survival distributions with respect to the treatment groups. A Cox proportional hazards model was used to adjust for covariates. These analyses were performed using SPSS® version 12.0.
The median overall survival of the entire population was 29.0 months [95% confidence interval (CI)=19.97-38.03]. The median survival was 14.0 months (95% CI=7.8-20.2) in RT group, 30.0 months (95% CI=21.4-38.6) in ACNU-CDDP group and 72.0 months (95% CI=52.0-91.9) in PCV group, respectively. There were significant differences in estimated survival among treatment groups (Fig. 1). PCV group was superior to both RT group (p=0.002) and ACNU-CDDP group (p=0.045) in survival gain. ACNU-CDDP group showed significant survival prolongation compared with RT group (p=0.039). If the extent of resection was considered for survival analysis significant differences in estimated survival among treatment groups were observed in both biopsy only population and in resection population. In biopsy only population, the median survival was 9.0 months (95% CI=2.7-15.3) in RT group, 28.0 months (95% CI=21.7-34.3) in ACNU-CDDP group and 47.0 months (95% CI=35.3-58.7) in PCV group, and their differences were significant (p=0.027 between PCV group and RT group and p=0.035 between ACNU-CDDP group and RT group) except for between PCV group and ACNU-CDDP group (p=0.2200). On the other hand, in resection population, the median survival was 23.0 months (95% CI=21.1-24.9) in RT group, 38.0 months (95% CI=25.4-50.6) in ACNU-CDDP group and 90.0 months (95% CI=47.1-132.8) in PCV group and significant difference in estimated survival among treatment groups was observed in only between PCV group and RT group (p=0.033). There was no significant differences in survival between PCV group and ACNU-CDDP group (p=0.192) and between ACNU-CDDP group and RT group (p=0.279) in resection population. Overall, add-on chemotherapy to radiotherapy was beneficial in survival gain compared with radiotherapy alone. Also, adjuvant PCV chemotherapy was more effective than neo-adjuvant ACNU-CDDP chemotherapy. In the multivariate analysis, the benefit of chemotherapy was confirmed once again along with good performance status (KPS ≥90) and extent of surgical resection (Table 2).
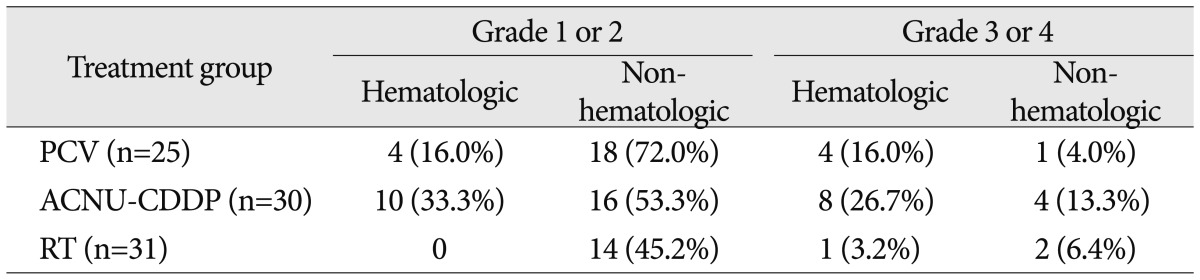
Toxicity associated with treatment was also analyzed (Table 3). In the PCV group, grade 3 or 4 toxicities were found in 5 patients (20%). Among them, 4 patients (16.0%) showed hematological toxicities and one patient (4.0%) showed non-hematological toxicity. In the ACNU-CDDP group, 12 patients (40%) experienced grade 3 or 4 toxicities which consist of 8 patients (26.7%) for hematological and 4 patients (13.3%) for non-hematological toxicities. There was a relatively lower rate of serious toxicities in RT group that only 3 patients (9.6%) experienced grade 3 or 4 toxicities.
The focal radiotherapy with a total dose of 60 Gy in 30 daily fractions has been the standard treatment for AA on the basis of level 1 evidence1). However, the benefit of chemotherapy in the treatment of AA remains controversial for many decades. Most prospective randomized trials on using chemotherapy for malignant gliomas failed to prove their efficacy10,12). Among the diverse efforts conducted using chemotherapy for malignant gliomas, carmustine and procarbazine showed modest benefit for survival outcome5,14). On the other hand, there also have been the concerns about considerable toxicities in applying chemotherapy to malignant glioma patients4,7). The problem of majority of previous studies is that they pooled both grades III or IV astrocytic tumors and analyzed the efficacy of treatment protocol for whole study population as a same group. However, there was need for separate study for AA was advocated after sub-analysis of the study population6,8). For example, European study testing adjuvant chemotherapy combining dibromodulcitol and carmustine given postoperatively to patients with newly diagnosed supratentorial malignant gliomas resulted in longer survival (8.1 months vs. 6.7 months) only in experimental arm of subgroup of 29% of non-glioblastoma patients6). In the present study, we confined the study population to newly diagnosed AA patients and confirmed the beneficial role of add-on chemotherapy over radiotherapy alone. Moreover, adjuvant chemotherapy with PCV after radiotherapy was proven to be advantageous compared with neo-adjuvant chemotherapy with ACNU-CDDP in terms of survival outcome and toxicity rate. The limitation of this study, however, should be taken into account as the retrospective data analysis could not avoid the selection bias at the stage of management decision. The treatment protocol between radiotherapy only and add-on chemotherapy to radiotherapy was rather randomly chosen according to the physicians' decision throughout the study period. However, chemotherapy regimen was changed according to the times that PCV group was enrolled in 1990s (from 1993 to 1999) and ACNU-CDDP group was enrolled in mostly early 2000s (from 1999 to 2004) (Table 1). The other shortcoming of the present study is that there is no consideration of detailed molecular markers such as 1p and 19q chromosomal deletion status, O6-methylguanine methyl transferase promoter methylation status or isocitrate dehydrogenase mutation status due to the unavailability of the tumor tissue.
Comparison of efficacy between PCV and nitrosourea also showed controversial results in previous studies. Analysis of Radiation Therapy Oncology Group (RTOG) database for newly diagnosed AA revealed no difference in survival after radiotherapy between adjuvant PCV group and adjuvant carmustine group11). This trial has been criticized for allowing different radiotherapy schedules and for giving a modified PCV regimen12). On the other hand, re-analysis of the Northern California Oncology Group protocol 6G61 study confirmed superior survival outcome of adjuvant PCV group (157.1 weeks) to that of adjuvant BCNU group (82.1 weeks) after radiotherapy only in AA patients8).
Although PCV chemotherapy has more tolerable toxicity profiles over ACNU-CDDP chemotherapy as shown in the present study, relatively high rate of significant toxicity in both regimens makes oncologist to hesitate over immediate application. If more tolerable chemotherapy regimen with comparable efficacy is proved to be recommendable in on-going trials such as TMZ in RTOG 9318 protocol or international CATNON trial, new era for standard therapy for AA including chemotherapy will come. Until then, adjuvant PCV after radiotherapy in selected AA patients with good performance is expected to give favorable survival outcome.
An application of chemotherapy before or after radiotherapy is beneficial compared with radiotherapy alone in prolonging the survival of patients with AA. Adjuvant PCV chemotherapy after radiotherapy is recommendable due to its superior survival outcome and better toxicity profile over neo-adjuvant ACNU-CDDP chemotherapy.
Acknowledgements
This study was supported by a grant of the Seoul National University Hospital Research Fund (04-2010-0470).
References
1. Bleehen NM, Stenning SP. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer. 1991; 64:769–774. PMID: 1654987.

2. Chang SM, Seiferheld W, Curran W, Share R, Atkins J, Choucair A, et al. Phase I study pilot arms of radiotherapy and carmustine with temozolomide for anaplastic astrocytoma (Radiation Therapy Oncology Group 9813) : implications for studies testing initial treatment of brain tumors. Int J Radiat Oncol Biol Phys. 2004; 59:1122–1126. PMID: 15234047.

3. Desjardins A, Reardon DA, Vredenburgh JJ. Current available therapies and future directions in the treatment of malignant gliomas. Biologics. 2009; 3:15–25. PMID: 19707392.
4. Eyre HJ, Eltringham JR, Gehan EA, Vogel FS, Al-Sarraf M, Talley RW, et al. Randomized comparisons of radiotherapy and carmustine versus procarbazine versus dacarbazine for the treatment of malignant gliomas following surgery : a Southwest Oncology Group Study. Cancer Treat Rep. 1986; 70:1085–1090. PMID: 3017551.
5. Green SB, Byar DP, Walker MD, Pistenmaa DA, Alexander E Jr, Batzdorf U, et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep. 1983; 67:121–132. PMID: 6337710.
6. Hildebrand J, Sahmoud T, Mignolet F, Brucher JM, Afra D. EORTC Brain Tumor Group. Adjuvant therapy with dibromodulcitol and BCNU increases survival of adults with malignant gliomas. Neurology. 1994; 44:1479–1483. PMID: 8058153.

7. Kim IH, Park CK, Heo DS, Kim CY, Rhee CH, Nam DH, et al. Radiotherapy followed by adjuvant temozolomide with or without neoadjuvant ACNU-CDDP chemotherapy in newly diagnosed glioblastomas : a prospective randomized controlled multicenter phase III trial. J Neurooncol. 2011; 103:595–602. PMID: 21052775.

8. Levin VA, Silver P, Hannigan J, Wara WM, Gutin PH, Davis RL, et al. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas : NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990; 18:321–324. PMID: 2154418.

9. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109. PMID: 17618441.

10. Medical Research Council Brain Tumor Working Party. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma : a Medical Research Council trial. J Clin Oncol. 2001; 19:509–518. PMID: 11208845.
11. Prados MD, Scott C, Curran WJ Jr, Nelson DF, Leibel S, Kramer S. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma : a retrospective review of radiation therapy oncology group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J Clin Oncol. 1999; 17:3389–3395. PMID: 10550132.

12. Stupp R, Reni M, Gatta G, Mazza E, Vecht C. Anaplastic astrocytoma in adults. Crit Rev Oncol Hematol. 2007; 63:72–80. PMID: 17478095.

13. Walker MD, Alexander E Jr, Hunt WE, MacCarty CS, Mahaley MS Jr, Mealey J Jr, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978; 49:333–343. PMID: 355604.

14. Walker MD, Green SB, Byar DP, Alexander E Jr, Batzdorf U, Brooks WH, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980; 303:1323–1329. PMID: 7001230.

15. Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999; 17:2762–2771. PMID: 10561351.
Fig. 1
Kaplan-Meier estimates of overall survival according to treatment group. Significant differences in estimated survival among treatment groups were observed (p=0.002 between PCV group and RT group; p=0.045 between PCV group and ACNU-CDDP group; p=0.039 between ACNU-CDDP group and RT group). PCV : procarbazine, lomustine and vincristine, ACNU-CDDP : nimustine-cisplatin, RT : radiotherapy.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download