Abstract
We report an unusual case of lateral medullary infarction after successful embolization of the vertebral artery dissecting aneurysm (VADA). A 49-year-old man who had no noteworthy previous medical history was admitted to our hospital with a severe headache. Computed tomography (CT) revealed a subarachnoid hemorrhage, located in the basal cistern and posterior fossa. Cerebral angiography showed a VADA, that did not involve the origin of the posterior inferior cerebellar artery (PICA). We treated this aneurysm via endovascular trapping of the vertebral artery distal to the PICA. After operation, CT revealed post-hemorrhagic hydrocephalus, which we resolved with a permanent ventriculoperitoneal shunt procedure. Postoperatively, the patient experienced transient mild hoarsness and dysphagia. Magnetic resonance image (MRI) showed a small infarction in the right side of the medulla. The patient recovered well, though he still had some residual symptom of dysphagia at discharge. Such an event is uncommon but can be a major clinical concern. Further investigation to reveal risk factors and/or causative mechanisms for the medullary infarction after successful endovascular trapping of the VADA are sorely needed, to minimize such a complication.
Stenosis or occlusion of the VA and cerebral aneurysms are commonly-associated vascular lesions. In patients with a vertebral artery dissecting aneurysm (VADA) resulting in subarachnoid hemorrhage (SAH), clinicians commonly advocate either proximal occlusion or trapping of the lesion is to prevent subsequent rebleeding1,2,5,11,14,17,21,23,27). Although certain studies have not established the long-term efficacy of aneurysm trapping by endovascular methods to prevent rebleeding7,25,26) the availability of endovascular surgery has increased the number of options for addressing a VADA during acute phase following SAH. This technique enables not only occlusion of the parent artery but also obliteration of the entire segment of the dissected site with coils more easily in posterior fossa, especially with regard to securing the distal side of the parent artery. When performing proximal occlusion or trapping of this lesion, the physician must make an attempt to spare the posterior inferior cerebellar artery (PICA), because ischemic symptoms of brain stem or cerebellum may occur leading to serious complications6-8,10,16,18,21,22,27). We report an unusual case of medullary infarction after successful endovascular trapping of the VADA.
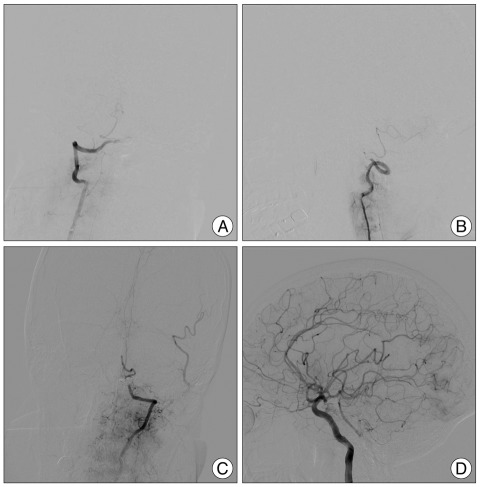
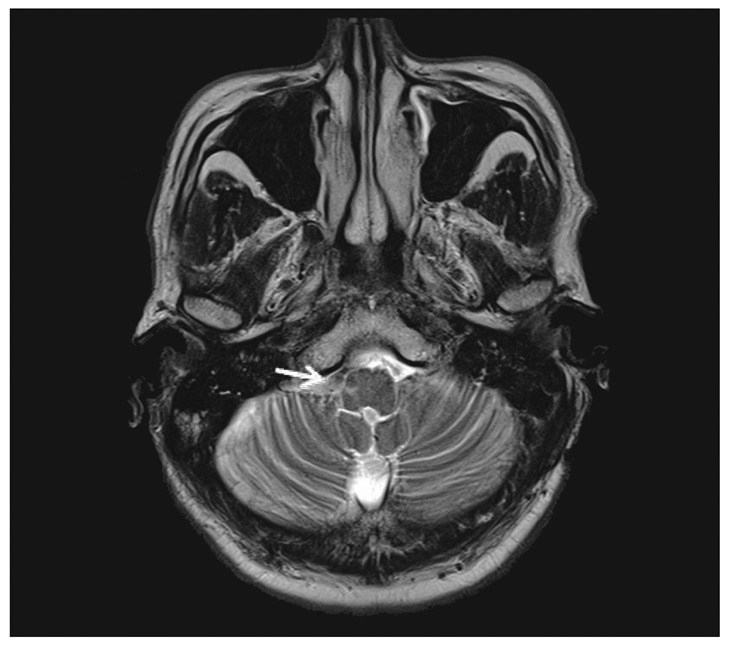
A 49-year-old man, who had no noteworthy previous medical history experienced the sudden onset of a severe headache followed by loss of consciousness. On neurological examination, he did not show lower cranial dysfunction. A CT scan revealed a SAH in the basal cistern and ventricular dilatation (Fig. 1A). Cerebral angiography on the day of admission demonstrated a fusiform VADA on the right side starting near the vertebrobasilar junction and proceeding to the origin of the PICA (Fig. 1B). Endovascular trapping of VADA was performed on a biplane angiographic unit (Integris BN 3000; Phillips Medical Systems, Best, the Netherlands). Procedure was performed after induction of general anesthesia without systemic heparinization. Endovascular trapping of VADA was performed using GDCs (Guglielmi detachable coils, Boston Scientific, Boston, MA, USA). After road mapping was performed using clear magnified images, a microcatheter (Excelsior SL-10; Boston Scientific, Boston, MA, USA) was carefully deployed into the VADA over the guidewire, and coils were then introduced. The aim of endovascular trapping was to obtain complete exclusion of the dissected segment including the VADA in accordance with securing the origin of the PICA (Fig. 2A, B). And, postembolization angiography showing the absence of retrograde filling of the dissecting aneurysm and a good blood flow of the basilar and left posterior cerebral artery through collateral flow from the posterior communicating artery (Fig. 2C, D). After operation, CT revealed the post-hemorrhagic hydrocephalus, which was resolved using a permanent ventriculoperitoneal shunt procedure. Postoperatively, the patient seemed to be recovering well but he experienced transient mild hoarsness and dysphagia. Magnetic resonance image showed an infarction on the right side of the medulla (Fig. 3). The patient recovered well and discharged with some residual symptom of dysphagia.
In patients with a dissecting aneurysm of the VA resulting in SAH, researchers and clinicians commonly advocate trapping of the lesion, to prevent subsequent rupture. Although the results of trapping are generally excellent, this procedure does not guarantee resolution of the ischemic event, lateral medullary infarction12,14,23).
Dissection leads to occlusion of perforating branches at the level of the aneurysm, with ischemic damage. Perforating arteries arising between the origin of the PICA and the vertebrobasilar junction are predominately of the short, circumflex type and terminate on the anterior and lateral surface of the medulla. The segment of the vertebral artery distal to the PICA origin gives rise to perforating arteries more frequently than the segment proximal to the PICA origin does. The syndrome associated with lateral medullary infarction may be caused by occlusion of either the PICA or the vertebral artery, but it is most commonly attributable to vertebral artery occlusion1,2,6-11,13,18,23). Fischer et al.3) note that 75% of cases of lateral medullary syndrome correlated with vertebral artery occlusion and only 12% had a PICA occlusion. Mahmood et al.15) report that perforators could arise from the vertebrobasilar arteries between approximately 14 mm proximal and 16 mm distal to the union. Numerous anatomic variations in the vascular anatomy of the PICA origin and the vertebrobasilar junction, along with the presence of delicate proximal perforators, make a standardized approach difficult. Although researchers cannot determine the exact ischemic mechanism, studies suggest compromised medullary penetrating arteries that arise from vertebral artery, thromboembolism during endovascular trapping of vertebral artery, and regional hemodynamic perfusion failure due to vertebral artery occlusion as probable causes of lateral medullary infarction4,7,19,20,24,28). If the dissection site occurs in the vertebral artery distal to the PICA, the physician can likely occlude many of the perforators via internal trapping at the time of any endovascular procedures, because this site is more likely to give rise to perforators. Endovascular internal trapping may be more reliable for the prevention of rebleeding; however, such trapping may precipitate lateral medullary syndrome or brain stem infarction6,13). The physician cannot always safely sacrifice the PICA's origin without producing an ischemic insult in the brain stem or cerebellum. In general, one can safely perform obliteration of the PICA at a point distal to the choroidal point. However, this is potentially dangerous if performed proximal to the choroidal point, because important branches to the brain stem and deep cerebellar nuclei lie proximal to the choroidal point1,7,25). The consequences of PICA occlusion are unpredictable and could range from a clinically silent occlusion to infarction of portions of brain stem or cerebellum. The perforating branches to the brain stem origin are from the PICA origin and the vertebrobasilar junction, and brain stem infarction may occur if the physician occludes large perforators.
To date, we think that there is no optimal method for the prevention of ischemic events and treatment of these regions in the event of dissecting aneurysm exists. Although the balloon occlusion test may, in fact, be helpful in assessing an ischemic event, its use is not always clinically feasible and safe1). Endovascular technique is a more suitable treatment for use in the acute phase because it is less invasive compared to open surgical clipping. Surgical clipping of VADA presents a risky and limitation due to the deep and narrow surgical field and possibility that retraction may damage the adjacent tissue and risk of injury to the vein. However, some cases of VADA are not amenable to concomitant parent vessel occlusion or trapping by endovascular methods in case of a high probability of blood flow disturbance such as an involvement of origin of PICA or perforators to brain stem. Advance in microsurgical techniques (vessel anastomosis) and in skull base surgery remains important, despite the evolution of endovascular techniques.
Although lateral medullary infarction after successful endovascular trapping of the VADA is uncommon, it could be of major clinical concern. Further investigation to reveal risk factors, vascular anatomy, and causative mechanisms for medullary infarction after successful endovascular treatment is sorely needed to minimize such a complication.
References
1. Ali MJ, Bendok BR, Tawk RG, Getch CC, Batjer HH. Trapping and revascularization for a dissecting aneurysm of the proximal posteroinferior cerebellar artery : technical case report and review of the literature. Neurosurgery. 2002; 51:258–262. discussion 262-263. PMID: 12182429.
2. Dinichert A, Rüfenacht DA, Tribolet N. Dissecting aneurysms of the posterior inferior cerebellar artery : report of four cases and review of the literature. J Clin Neurosci. 2000; 7:515–520. PMID: 11029232.

3. Fisher CM, Karnes WE, Kubik CS. Lateral medullary infarction-the pattern of vascular occlusion. J Neuropathol Exp Neurol. 1961; 20:323–379. PMID: 13699936.

4. García-García J, Ayo-Martín O, Segura T. Lateral medullary syndrome and ipsilateral hemiplegia (Opalski syndrome) due to left vertebral artery dissection. Arch Neurol. 2009; 66:1574–1575. PMID: 20008668.

5. Giannopoulos S, Markoula S, Kosmidou M, Pelidou SH, Kyritsis AP. Lateral medullary ischaemic events in young adults with hypoplastic vertebral artery. J Neurol Neurosurg Psychiatry. 2007; 78:987–989. PMID: 17702781.

6. Hamada J, Kai Y, Morioka M, Yano S, Todaka T, Ushio Y. Multimodal treatment of ruptured dissecting aneurysms of the vertebral artery during the acute stage. J Neurosurg. 2003; 99:960–966. PMID: 14705721.

7. Iihara K, Sakai N, Murao K, Sakai H, Higashi T, Kogure S, et al. Dissecting aneurysms of the vertebral artery : a management strategy. J Neurosurg. 2002; 97:259–267. PMID: 12186451.
8. Iwabuchi S, Yokouchi T, Kimura H, Ueda M, Samejima H. Rupture of a large vertebral artery aneurysm following proximal occlusion. Interv Neuroradiol. 2005; 11:51–58. PMID: 20584435.

9. Jao T, Liu HM, Tang SC, Jeng JS. Dissection of the posterior inferior cerebellar artery in a young adult with cerebellar infarct. Acta Neurol Taiwan. 2008; 17:243–247. PMID: 19280868.
10. Jeon SG, Kwon do H, Ahn JS, Kwun BD, Choi CG, Jin SC. Detachable coil embolization for saccular posterior inferior cerebellar artery aneurysms. J Korean Neurosurg Soc. 2009; 46:221–225. PMID: 19844622.

11. Kakino S, Ogasawara K, Kubo Y, Otawara Y, Tomizuka N, Suzuki M, et al. Treatment of vertebral artery aneurysms with posterior inferior cerebellar artery-posterior inferior cerebellar artery anastomosis combined with parent artery occlusion. Surg Neurol. 2004; 61:185–189. discussion 189. PMID: 14751640.

12. Kwon M, Lee JH, Kim JS. Dysphagia in unilateral medullary infarction : lateral vs medial lesions. Neurology. 2005; 65:714–718. PMID: 16157904.

13. Lee JM, Kim TS, Joo SP, Yoon W, Choi HY. Endovascular treatment of ruptured dissecting vertebral artery aneurysms--long-term follow-up results, benefits of early embolization, and predictors of outcome. Acta Neurochir (Wien). 2010; 152:1455–1465. PMID: 20467760.

14. Lewis SB, Chang DJ, Peace DA, Lafrentz PJ, Day AL. Distal posterior inferior cerebellar artery aneurysms : clinical features and management. J Neurosurg. 2002; 97:756–766. PMID: 12405360.

15. Mahmood A, Dujovny M, Torche M, Dragovic L, Ausman JI. Microvascular anatomy of foramen caecum medullae oblongatae. J Neurosurg. 1991; 75:299–304. PMID: 2072169.

16. Mizushima H, Sasaki K, Kunii N, Nishino T, Jinbo H, Abe T, et al. Dissecting aneurysm in the proximal region of the posterior inferior cerebellar artery presenting as Wallenberg's syndrome--case report. Neurol Med Chir (Tokyo). 1994; 34:307–310. PMID: 7519754.

17. Nguyen TN, Roy D, Guilbert F, Raymond J, Weill A. Endovascular trapping of a vertebral artery segment to control PICA origin tearing. J Neuroimaging. 2008; 18:418–421. PMID: 18302643.

18. Ogasawara K, Kubo Y, Tomitsuka N, Sasoh M, Otawara Y, Arai H, et al. Treatment of vertebral artery aneurysms with transposition of the posterior inferior cerebellar artery to the vertebral artery combined with parent artery occlusion. Technical note. J Neurosurg. 2006; 105:781–784. PMID: 17121146.

19. Okuchi K, Watabe Y, Hiramatsu K, Tada T, Sakaki T, Kyoi K, et al. [Dissecting aneurysm of the vertebral artery as a cause of Wallenberg's syndrome]. No Shinkei Geka. 1990; 18:721–727. PMID: 2215865.
20. Paliwal VK, Kalita J, Misra UK. Dysphagia in a patient with bilateral medial medullary infarcts. Dysphagia. 2009; 24:349–353. PMID: 19115072.

21. Peluso JP, van Rooij WJ, Sluzewski M, Beute GN, Majoie CB. Posterior inferior cerebellar artery aneurysms : incidence, clinical presentation, and outcome of endovascular treatment. AJNR Am J Neuroradiol. 2008; 29:86–90. PMID: 17928380.

22. Porto FH, da Silva SP, Orsini M, de Freitas MR, de Freitas GR. Hemimedullary infarct with ipsilateral hemiplegia : a vertebral artery dissection syndrome? J Neurol Sci. 2009; 278:135–137. PMID: 19108851.

23. Redekop G, TerBrugge K, Willinsky R. Subarachnoid hemorrhage from vertebrobasilar dissecting aneurysm treated with staged bilateral vertebral artery occlusion : the importance of early follow-up angiography : technical case report. Neurosurgery. 1999; 45:1258–1262. discussion 1262-1263. PMID: 10549948.
24. Shioya H, Kikuchi K, Suda Y, Shindo K. [Atypical Wallenberg's syndrome due to spontaneous vertebral arterial dissection : case report]. No To Shinkei. 2004; 56:519–524. PMID: 15328842.
25. Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990; 72:183–188. PMID: 2404089.

26. Yamaura I, Tani E, Yokota M, Nakano A, Fukami M, Kaba K, et al. Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Guglielmi detachable coils. J Neurosurg. 1999; 90:853–856. PMID: 10223450.

27. Yasui T, Komiyama M, Nishikawa M, Nakajima H. Subarachnoid hemorrhage from vertebral artery dissecting aneurysms involving the origin of the posteroinferior cerebellar artery : report of two cases and review of the literature. Neurosurgery. 2000; 46:196–200. discussion 200-201. PMID: 10626950.

28. Yeh HF, Seak CJ, Chiu TF, Chang YC. Traumatic vertebral artery dissection and Wallenberg syndrome after a motorcycle collision. Am J Emerg Med. 2009; 27:131e1–e3. PMID: 19041559.

Fig. 1
A : Computed tomography axial image demonstrating a diffuse subarachnoid hemorrhage in the basal cistern. B : Preoperative right vertebral angiography showing a typical dissecting aneurysm of the vertebral artery distal to the posterior inferior cerebellar artery.

Fig. 2
Postembolization right vertebral angiography, anteroposterior (A) and lateral (B) view, demonstrating complete occlusion of the right vertebral artery and good patency of the right posteroinferior cerebellar artery. Postembolization left vertebral angiography (C) demonstrating supply of lower part of the basilar artery and the left posterior inferior cerebellar artery and the absence of retrograde filling of the dissecting aneurysm. Left internal carotid angiography (D) showing a good blood flow of the basilar and the left posterior cerebral artery through collateral flow from the posterior communicating artery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download