Abstract
Central nervous system (CNS) sparganosis is a rare parasitic infestation caused by ingestion of the raw or inadequately cooked snakes or frogs. Sparganum is well known for its ability of migrating though the tissue, therefore, it can cause various neurological symptoms if it involves neurological systems. A 51-year-old male patient visited our department of neurosurgery complaining of the motor weakness and radiating pain on both upper extremities over 4 months. He had a history of ingesting raw snakes untill his late twenties. The magnetic resonance (MR) images of cervical spine revealed an intramedullary ill-defined enhancing lesion with the aggregated cysts in the upper cervical spinal cord. Under presumptive diagnosis of sparganosis, we took brain MR image. The brain MR images revealed the signal change in right fronto-temporal lobe suggesting the trajectory of parasitic migration via ventricular systems. He underwent a midline myelotomy and granuloma removal followed by the posterior laminoplasty. Pathologic findings showed inflammatory changes and necrosis with keratinized tissue suggesting the CNS sparganosis. We report an uncommon case of CNS sparganosis migrated from the brain to the spinal cord with literature review.
It is well known that central nervous system (CNS) sparganosis is a rare parasitic infestation caused by Spirometra mansoni. To our knowledge, a case with the contralateral migration of cerebral sparganosis has been reported, but case of migration from the brain to the spinal cord has not been reported yet1,2).
We report a case with CNS sparganosis, which has been migrated from the right fronto-temporal lobe to the cervical spinal cord.
A 51-year-old man visited our hospital complaining of motor weakness and radiating pain to both upper extremities for 4 months. He had a history of ingestion of raw snakes until the late twenties of his age.
Physical examination of patient was not remarkable. But, the neurological evaluation revealed that motor power was 3/5 in the left upper extremity, however muscle atrophy was not observed. He had impaired sensation on the both C4, 5, 6 dermatomes.
Laboratory assessment including complete blood cell count, erythrocyte sedimentation rate, blood biochemistry and urine analysis were within normal range. Tumor markers, anti-HIV antibodies, hepatitis B surface antigen and anti-hepatitis C virus antibodies were negative.
The leukocyte count and the level of glucose were normal in cerebrospinal fluid (CSF). But CSF albumin level was 30.78 mg/dL (normal range 12.7-13.4 mg/dL) and IgG-CSF was 7.93 mg/dL (normal range 0.48-5.2 mg/dL). And the enzyme-linked immunosorbent assay for sparganosis-specific antibody was positive in CSF but negative in serum.
Magnetic resonance (MR) images of cervical spine revealed that intramedullary ill-defined enhancing lesions with internal multifocal cystic change surrounded by swelling from lower medulla to C6 level (Fig. 1). Brain enhanced computed tomography (CT) scans revealed that white matter hypodensity with adjacent ventricular dilatation and irregular enhanced nodules were seen. White matter degeneration with cortical atrophy and ipsilateral ventricular dilatation were also revealed in brain MR images (Fig. 2).
The patient underwent a laminotomy and midline myelotomy. On the operative view, the edematous yellowish cord was seen at adjacent to the mass. The lobulated, yellowish mass was removed carefully but the live worm was not detected.
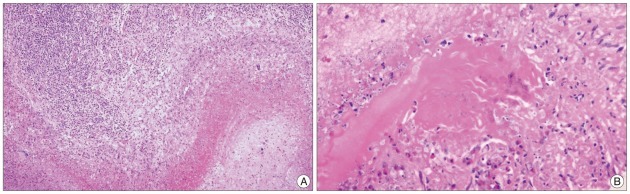
Histological examination of resected lesion showed that granulomatous inflammation with degenerated necrotic tissue containing numerous keratinization and infiltrations of intense mononuclear lymphoplasmacyte (Fig. 3).
The patient received corticosteroid therapy on immediate postoperative period, and paraesthesia on the right arm was improved. However, he complained of the paraesthesia on the left arm and both motor weakness persistently.
Sparganosis is an infection caused by a tapeworm larva, called sparganum, of the genus Spirometra, a cestode tapeworm closely related to Diphyllobothrium1). Human cerebral sparganosis is rare parasitic infestation of the CNS. Human infection seems to occur accidentally by ingestion of polluted water containing cyclops in which tapeworm eggs mature into procercoid larva, by ingestion of raw or inadequately cooked flesh of snakes or frogs, and by applying the flesh of an infected intermediate host as a poultice to a wound4,5).
The route of infection to the CNS is not yet known, but the sparganum may unintentionally migrate through the foramen of the skull base and vertebral column along the loose connective tissue around the vessels or nerves.
The symptoms and signs of cerebral sparganosis are non-specific and except for a past history of eating raw meat, there is no precise clinical information that would indicate the infestation1). The complete removal of a worm including its scolex, is therefore essential for a favorable clinical course1).
The radiological findings of cerebral sparganosis are edema and degeneration of the white matter, which show hypodensity on the unenhanced CT images, hypointensity on T1-weighted images, and hyperintensity on T2-weighted images7). The most important and characteristic finding is the tunnel sign on the postcontrast MR images7). The tunnel sign appeares hypointense on T1-weighted images and slightly hyperintense or isointense on T2-weighted images7).
Characteristic pathologic finging of sparganosis is variable degrees of mummification of worms found in the center of granulomatous lesions. Rengarajan et al.6) reported that histological examination of the resected lesion showed a large well-formed abscess with dense inflammatory infiltration walled in by gliotic wall. Within the abscess cavity, cross-sections of the larval form of cestode Spirometra mansoni, entrapped in inflammatory cells was found.
Sparganosis in human can invade not only the subcutaneous tissue or muscles but in the abdominal cavity, genitourinary tract, eye, spinal canal, and brain. Kudesia et al.3) reported that sparganosis usually appears as slowly growing and migratory subcutaneuous nodules, though the parasite can be found anywhere in the body, including nervous system. Migration of sparganosis in CNS is one of the characteristics of sparganosis. Kim et al.2) mentioned that the migration could be explained obviously with the fluid attenuated inversion recovery image, which showed that the migration route had a high signal intensity. Song et al.7) reported that tunnel represented the moving track of a migrating worm, appearing as a solid or hollow tube, which corresponded to the inflammatory granulomas according to the postoperative pathologic examination. In our case, the cervical MR images revealed intramedullary ill-defined tubular enhancing lesions in the upper cervical cord. The brain MR images revealed inflammatory change on the right fronto-temporal lobe area suggesting the trajectory of parasitic migration via ventricular system. So, we consider that the tunnel like cavities from brain to spinal cord reveal the trajectory of migration for lava of tapeworms.
References
1. Kim DG, Paek SH, Chang KH, Wang KC, Jung HW, Kim HJ, et al. Cerebral sparganosis : clinical manifestations, treatment, and outcome. J Neurosurg. 1996; 85:1066–1071. PMID: 8929496.
2. Kim IY, Jung S, Jung TY, Kang SS, Chung TW. Contralateral migration of cerebral sparganosis through the splenium. Clin Neurol Neurosurg. 2007; 109:720–724. PMID: 17630134.

3. Kudesia S, Indira DB, Sarala D, Vani S, Yasha TC, Jayakumar PN, et al. Sparganosis of brain and spinal cord : unusual tapeworm infestation (report of two cases). Clin Neurol Neurosurg. 1998; 100:148–152. PMID: 9746305.

4. Ou Q, Li SJ, Cheng XJ. Cerebral sparganosis : a case report. Biosci Trends. 2010; 4:145–147. PMID: 20592465.
5. Park JH, Park YS, Kim JS, Roh SW. Sparganosis in the lumbar spine : report of two cases and review of the literature. J Korean Neurosurg Soc. 2011; 49:241–244. PMID: 21607186.

6. Rengarajan S, Nanjegowda N, Bhat D, Mahadevan A, Sampath S, Krishna S. Cerebral sparganosis : a diagnostic challenge. Br J Neurosurg. 2008; 22:784–786. PMID: 18661311.
7. Song T, Wang WS, Zhou BR, Mai WW, Li ZZ, Guo HC, et al. CT and MR characteristics of cerebral sparganosis. AJNR Am J Neuroradiol. 2007; 28:1700–1705. PMID: 17885230.

Fig. 1
Cervical magnetic resonance images. A : Irregular high signal intensity is shown from medulla oblongata to C7 level. B and C : Intramedullary ill-defined enhancing lesion with internal multifocal cystic change or aggregated cysts in the upper cervical spinal cord was seen.
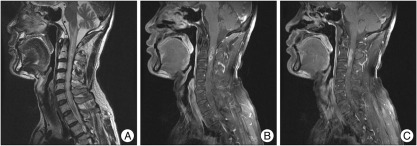
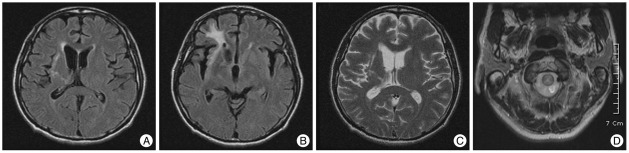
Fig. 2
A : The FLAIR images of MRI show high signal intensity on right fronto-temporal lobe. B and C : A high signal intensity on from basal ganglia and thalamus to ventricle is suggested from the trajectory of parasitic migration via the ventricular system. D : T2-weighted images show high signal intensity on the citerna magna. FLAIR : fluid attenuated inversion recovery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download