Abstract
Objective
To evaluate the interactions among differentially expressed autophagy and mitophagy markers in subarachnoid hemorrhage (SAH) patients with delayed cerebral ischemia (DCI).
Methods
The expression data of autophagy and mitophagy-related makers in the cerebrospinal fluid (CSF) cells was analyzed by real-time reverse transcription-polymerase chain reaction and Western blotting. The markers included death-associated protein kinase (DAPK)-1, BCL2 interacting protein 3 like (BNIP3L), Bcl-1 antagonist X, phosphatase and tensin homolog-induced kinase (PINK), Unc-51 like autophagy activating kinase 1, nuclear dot protein 52, and p62. In silico functional analyses including gene ontology enrichment and the protein-protein interaction network were performed.
Results
A total of 56 SAH patients were included and 22 (38.6%) of them experienced DCI. The DCI patients had significantly increased mRNA levels of DAPK1, BNIP3L, and PINK1, and increased expression of BECN1 compared to the non-DCI patients. The most enriched biological process was the positive regulation of autophagy, followed by the response to mitochondrial depolarization. The molecular functions ubiquitin-like protein ligase binding and ubiquitin-protein ligase binding were enriched. In the cluster of cellular components, Lewy bodies and the phagophore assembly site were enriched. BECN1 was the most connected gene among the differentially expressed markers related to autophagy and mitophagy in the development of DCI.
Delayed cerebral ischemia (DCI) is still a significant risk factor for poor neurologic outcomes in patients with SAH [21]. Previous reports on the relationship with DCI mainly studied three mechanisms including cerebral vasospasm, cortical spreading depolarization, and microthrombosis [18]. Nevertheless, the exact mechanism is still unknown, and thus, efforts are ongoing to identify other mechanisms of the pathogenesis of DCI.
Autophagy refers to a homeostatic cellular process by the degradation of dysfunctional organelles and superfluous proteins [11,25]. In the SAH research area, the main focus of autophagy has been its possible association with early brain injury (EBI) in rodent models. Lee et al. [15] reported that autophagosomes and autolysosomes in neurons increased markedly from the first day of an SAH. In addition, the high conversion of microtubule-associated protein light chain-3 (LC3)-I to LC3-II and increased expression of BECN1 and cathepsin-D were observed. During the acute phase, rapamycin, which is an autophagy activator, decreased in brain edema, cortical apoptosis, and blood-brain barrier impairment compared to vehicle-treated SAH rats [26]. Mitophagy is selective autophagy that maintains health mitochondria by the selective removal of dysfunctional or damaged mitochondria [16,28]. Wu et al. [28] reported that the knockdown of phosphatase and tensin homolog-induced kinase 1 (PINK1) and parkin was associated with the intracellular accumulation of mitochondrial fragments and damaged mitochondria via reduced mitophagy in endothelial cells. Cao et al. [1] also showed the neuroprotective effect of inhibiting mitophagy-associated nod-like receptor protein 3 inflammasomes against EBI after SAH. Compared to EBI, few studies have investigated the possible role of mitophagy in the development of DCI. Chou et al. [4] reported that higher mitochondrial membrane potential was associated with favorable functional outcomes in SAH patients. Youn et al. [30] reported that DCI patients had significantly decreased mitochondria membrane potential in CSF cells than non-DCI patients. These results suggest a possible association of mitochondrial dysfunction with DCI pathogenesis. A follow-up investigation showed that mitochondrial dysfunction associated with autophagy and mitophagy might have a role in DCI development [31]. The DCI patients showed a higher expression of death-associated protein kinase (DAPK)-1, BCL2 interacting protein 3 like (BNIP3L), and PINK1 than the non-DCI patients. In particular, DAPK1 was the most significant one among markers differentially expressed between those with DCI and those without DCI.
Bioinformatics analysis has been increasingly reported in various medical conditions in anticipation of suggesting new insight into the disease pathogenesis. An epigenome-wide association study found that hypermethylation of the INSR gene (cg00441765) and the CDHR5 gene (cg11464053) was closely related to DCI [13]. This result indicated a simple difference in the methylation frequencies of CpG sites according to DCI. Subsequent bioinformatics analysis revealed additional information on various biological processes (BP) and hub genes associated with DCI, which existing analysis methods do not provide. Here, using bioinformatics analysis of the raw data, we further evaluated the interactions among the differentially expressed autophagy and mitophagy biomarkers of CSF cells associated with DCI.
This study was approved by the Institutional Review Board (No. 2017-9, 2018-6, and 2019-6) of the hospital and informed consent was obtained from the patients or their relatives.
The original dataset was derived from an ongoing study entitled “The First Korean Stroke Genetics Association Research” of the five university hospitals that prospectively collected the genome and protein databank of patients with various cerebrovascular diseases since March 2015 [12,17]. In this databank, the CSF samples of SAH patients were investigated to determine whether mitochondrial dysfunction associated with autophagy and mitophagy may be related to DCI following SAH (Fig. 1). The expression of the markers was measured using real-time reverse transcription-polymerase chain reaction (qRT-PCR) and Western blotting. The markers measured by qRT-PCR were 1) DAPK-1; 2) BNIP3L; 3) Bcl-1 antagonist X (BAX); 4) PINK1; 5) Unc-51 like autophagy activating kinase 1 (ULK1); and 6) nuclear dot protein 52 (NDP52). The markers measured by Western blotting were 1) BECN1 (autophagy executor gene); and 2) p62 (autophagy adaptor protein) [5,24,31].
Gene ontology (GO) enrichment (https://tools.dice-database.org/GOnet) and Kyoto Encyclopedia of Genes and Genomes pathway (https://www.genome.jp/kegg/mapper/color.html) analyses were carried out using the visualization tools [32]. GO terms with a p-value of less than 0.05 were defined as significantly enriched. Protein-protein interaction (PPI) network analysis was performed using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING). The minimum required interaction score was set to the highest confidence of 0.7 [19,23].
Data on CSF cells obtained from 56 SAH patients were selected for the analysis. Among them, DCI was observed in 22 patients (38.6%). A flow chart of the study is presented in Supplementary Fig. 1. Transmission electron microscopy showed various mitochondrial conditions in DCI such as damaged mitochondria and disarrayed cristae with autophagic vacuoles and lysosomes (Fig. 1). Among the various markers, DCI patients exhibited significantly increased mRNA levels of DAPK1, BNIP3L, and PINK1, and protein expression of BECN1 with p62 degradation than compared to the non-DCI patients.
GO enrichment analysis was performed for BP, molecular function (MF), and cellular components (CC). The most enriched BP functions were the positive regulation of autophagy, followed by the response to mitochondrial depolarization, the positive regulation of apoptosis, and mitochondrial autophagy in the development of DCI (Table 1 and Fig. 2A). In MF, DCI was associated with ubiquitin-like protein ligase binding and ubiquitin-protein ligase binding (Fig. 2B). In the cluster of CCs, Lewy bodies and the phagophore assembly site were the most enriched in DCI patients (Fig. 2C). Three and four of the differentially expressed genes are marked in red in the autophagy and mitophagy pathways in DCI development in Fig. 3, respectively. PPI analysis revealed that BECN1 was the most connected gene among the differentially expressed markers associated with autophagy and mitophagy in SAH patients with DCI (Fig. 2D).
Few studies have investigated mitophagy associated with DCI in SAH patients, although mitophagy research has been actively conducted in other brain diseases. Conventional mitophagy is regulated by the PINK1/parkin pathway [6]. In the damaged mitochondria of patients with autosomal recessive Parkinson’s disease, PINK1 is expressed on the outer membrane during activated E3 ubiquitin ligase activity, which recruits parkin. This selective lysosomal removal was followed by parkin-mediated polyubiquitination [6,20]. Parkin-mediated mitophagy was also involved in the cardiac stress-response mechanism [7]. Kubli et al. [14] reported that parkin-deficient mice showed increased mitochondrial dysfunction associated with decreased mitophagy myocardial infarction. However, alternative parkin-independent mitophagy or autophagy adapter proteins are also involved in mitophagy [6]. Phosphorylated Unc-51-like kinase 1, which interacts with Ras-related protein 9 at serine 179 followed by complex formation with Rip 1 and dynamic-related protein (Drp) 1 were observed in the heart in ischemic and glucose-deprived states. After phosphorylated Drp1 was sequestrated, the lysosomal degradation of mitochondria was observed [6,22]. In SAH patients, a higher expression of PINK1 seemed to be associated with DCI. And DAPK1 and BNIP3L were significantly higher in DCI patients than in non-DCI patients. Our additional bioinformatics analysis showed that the positive regulation of autophagy was mostly enriched, followed by the response to mitochondrial depolarization in BP in DCI development. DAPK1, BNIP3L, BECN1, and PINK1 were involved in the positive regulation of autophagy, and BECN1, PINK1, and p62 in the response to mitochondrial depolarization. PPI analysis also demonstrated that BECN1 was the most connected gene among the highly expressed biomarkers in DCI development. BECN1 is expressed within cytoplasmic structures such as mitochondria and the endoplasmic reticulum and contains three domains including the Bcl-2 homology motif, the coiled-coil domain, and the evolutionarily conserved domain (ECD) [8]. BECN1 interacted with PI3KCIII/Vps34 via ECD and positively regulated the autophagy reaction [8]. Diabetic mice showed increased autophagosome formation and autophagic flux with a higher expression of BECN1, microtubule-associated protein LC3-II, and a lower expression of p62 [27]. BECN1 has been suggested as a cancer target for anti-tumor agents that enhance BECN1 activity. Based on these findings, it is necessary to investigate which specific mechanisms and proteins can be therapeutic targets for DCI in the future.
Several clinical variables such as hypertension and sex differences may affect autophagy and mitophagy in DCI. In DCI patients, blood pressure was maintained at high levels to increase blood flow to the brain. Hypertension itself may affect autophagy and mitophagy, although the exact mechanism remains undetermined. Increased BNIP3 expression was correlated with pressure overload in heart failure [2]. Givvimani et al. [10] reported that mitophagy inhibitor ameliorated heart failure by decreasing pressure overload concomitant with the decreased expression of LC3 and p62. Gender can influence not only DCI occurrence but also autophagy and the mitophagy process. The rate of DCI was significantly higher in females compared to males [9]. Chen et al. [3] reported that female rats exhibited a higher ratio of LC3B to LC3A in a cardiac ischemia-reperfusion model. Regarding the two pro-apoptotic proteins of phosphor-p38 and Bax, male rats showed higher levels of phosphor-p38, and female rats showed lower levels of Bax after ischemic-reperfusion. Female ovariectomized rats exhibited an increased expression of LC3 and BECN1 with decreased p62 [29], suggesting that autophagy and mitophagy may vary depending upon sex hormones. Although, there were no significant differences in hypertension and gender between the DCI and non-DCI groups in the original dataset, additional bioinformatics analysis adjusting for possible clinical variables that may affect the results is required in a large SAH population.
This study had some limitations. First, the relatively small number of enrolled patients in the original data is a concern, although this was the first bioinformatics analysis of autophagy and mitophagy markers related to DCI. Second, we used some representative biomarkers of autophagy and mitophagy. Thus, in addition to the biomarkers we studied, follow-up studies are needed, including many biomarkers to get more accurate results. Third, bioinformatics results are largely dependent upon the quality of the raw data. To address this issue, it is necessary to perform the study considering various medical conditions that may affect the outcomes.
We comprehensively analyzed autophagy and mitophagy markers associated with DCI using bioinformatics analysis. These attempts can be helpful for understating DCI pathogenesis. We also identified a hub gene of BECN1, which is expected to be a therapeutic target for future DCI treatment in patients with SAH.
Notes
ACKNOWLEDGMENTS
This research was supported by the National Research Foundation of Korea funded by the Ministry of Education (2020R1l1A3070726) and Hallym Research Fund.
Supplementary materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2021.0169.
Supplementary Fig. 1.
Flow chart of the study design. SAH : subarachnoid hemorrhage, qRT-PcR : real-time reverse transcription polymerase chain reaction, GO : gene ontology, KEGG : Kyoto Encyclopedia of Genes and Genomes, PPI : protein-protein interaction.
References
1. Cao S, Shrestha S, Li J, Yu X, Chen J, Yan F, et al. Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of NLRP3 inflammasome activation. Sci Rep. 7:2417. 2017.

2. Chaanine AH, Jeong D, Liang L, Chemaly ER, Fish K, Gordon RE, et al. JNK modulates FOXO3a for the expression of the mitochondrial death and mitophagy marker BNIP3 in pathological hypertrophy and in heart failure. Cell Death Dis. 3:265. 2012.

3. Chen C, Hu LX, Dong T, Wang GQ, Wang LH, Zhou XP, et al. Apoptosis and autophagy contribute to gender difference in cardiac ischemia-reperfusion induced injury in rats. Life Sci. 93:265–270. 2013.

4. Chou SH, Lan J, Esposito E, Ning M, Balaj L, Ji X, et al. Extracellular mitochondria in cerebrospinal fluid and neurological recovery after subarachnoid hemorrhage. Stroke. 48:2231–2237. 2017.

5. Comincini S, Manai F, Meazza C, Pagani S, Martinelli C, Pasqua N, et al. Identification of autophagy-related genes and their regulatory miRNAs associated with celiac disease in children. Int J Mol Sci. 18:391. 2017.

6. Dhingra R, Rabinovich-Nikitin I, Kirshenbaum LA. Ulk1/Rab9-mediated alternative mitophagy confers cardioprotection during energy stress. J Clin Invest. 129:509–512. 2019.

8. Fu LL, Cheng Y, Liu B. Beclin-1: autophagic regulator and therapeutic target in cancer. Int J Biochem Cell Biol. 45:921–924. 2013.

9. Germans MR, Jaja BNR, de Oliviera Manoel AL, Cohen AH, Macdonald RL. Sex differences in delayed cerebral ischemia after subarachnoid hemorrhage. J Neurosurg. 129:458–464. 2018.

10. Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC. Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS One. 7:e32388. 2012.

11. Ho WM, Akyol O, Reis H, Reis C, McBride D, Thome C, et al. Autophagy after subarachnoid hemorrhage: can cell death be good? Curr Neuropharmacol. 16:1314–1319. 2018.

12. Hong EP, Kim BJ, Cho SS, Yang JS, Choi HJ, Kang SH, et al. Genomic variations in susceptibility to intracranial aneurysm in the Korean population. J Clin Med. 8:275. 2019.

13. Kim BJ, Kim Y, Youn DH, Park JJ, Rhim JK, Kim HC, et al. Genome-wide blood DNA methylation analysis in patients with delayed cerebral ischemia after subarachnoid hemorrhage. Sci Rep. 10:11419. 2020.

14. Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, et al. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 288:915–926. 2013.

15. Lee JY, He Y, Sagher O, Keep R, Hua Y, Xi G. Activated autophagy pathway in experimental subarachnoid hemorrhage. Brain Res. 1287:126–135. 2009.

16. Li J, Lu J, Mi Y, Shi Z, Chen C, Riley J, et al. Voltage-dependent anion channels (VDACs) promote mitophagy to protect neuron from death in an early brain injury following a subarachnoid hemorrhage in rats. Brain Res. 1573:74–83. 2014.

17. Park JJ, Kim BJ, Youn DH, Choi HJ, Jeon JP. A preliminary study of the association between SOX17 gene variants and intracranial aneurysms using exome sequencing. J Korean Neurosurg Soc. 63:559–565. 2020.

18. Park JJ, Kim Y, Chai CL, Jeon JP. Application of near-infrared spectroscopy for the detection of delayed cerebral ischemia in poor-grade subarachnoid hemorrhage. Neurocrit Care. 2021; [Epub ahead of print].

19. Penn AM, Saly V, Trivedi A, Lesperance ML, Votova K, Jackson AM, et al. Differential proteomics for distinguishing ischemic stroke from controls: a pilot study of the SpecTRA project. Transl Stroke Res. 9:590–599. 2018.

20. Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 85:257–273. 2015.

21. Rhim JK, Youn DH, Kim BJ, Kim Y, Kim S, Kim HC, et al. The role of consecutive plasma copeptin levels in the screening of delayed cerebral ischemia in poor-grade subarachnoid hemorrhage. Life (Basel). 11:274. 2021.

22. Saito T, Nah J, Oka SI, Mukai R, Monden Y, Maejima Y, et al. An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. J Clin Invest. 129:802–819. 2019.

23. Sang L, Wang XM, Xu DY, Zhao WJ. Bioinformatics analysis of aberrantly methylated-differentially expressed genes and pathways in hepatocellular carcinoma. World J Gastroenterol. 24:2605–2616. 2018.

24. Seto S, Tsujimura K, Horii T, Koide Y. Autophagy adaptor protein p62/SQSTM1 and autophagy-related gene Atg5 mediate autophagosome formation in response to Mycobacterium tuberculosis infection in dendritic cells. PLoS One. 8:e86017. 2013.

25. Wang X, Fang Y, Huang Q, Xu P, Lenahan C, Lu J, et al. An updated review of autophagy in ischemic stroke: from mechanisms to therapies. Exp Neurol. 340:113684. 2021.

26. Wang Z, Shi XY, Yin J, Zuo G, Zhang J, Chen G. Role of autophagy in early brain injury after experimental subarachnoid hemorrhage. J Mol Neurosci. 46:192–202. 2012.

27. Wu Q, Xiang M, Wang K, Chen Z, Long L, Tao Y, et al. Overexpression of p62 induces autophagy and promotes proliferation, migration and invasion of nasopharyngeal carcinoma cells through promoting ERK signaling pathway. Curr Cancer Drug Targets. 20:624–637. 2020.

28. Wu W, Xu H, Wang Z, Mao Y, Yuan L, Luo W, et al. PINK1-parkin-mediated mitophagy protects mitochondrial integrity and prevents metabolic stress-induced endothelial injury. PLoS One. 10:e0132499. 2015.

29. Yang Y, Zheng X, Li B, Jiang S, Jiang L. Increased activity of osteocyte autophagy in ovariectomized rats and its correlation with oxidative stress status and bone loss. Biochem Biophys Res Commun. 451:86–92. 2014.

30. Youn DH, Kim BJ, Kim Y, Jeon JP. Extracellular mitochondrial dysfunction in cerebrospinal fluid of patients with delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 33:422–428. 2020.

Fig. 1.
Transmission electron microscopy showing damaged mitochondria, autophagic vacuoles, lysosomes, and peroxisomes in the cerebrospinal fluid cells of patients with subarachnoid hemorrhage. Scale bar, 2 μm.
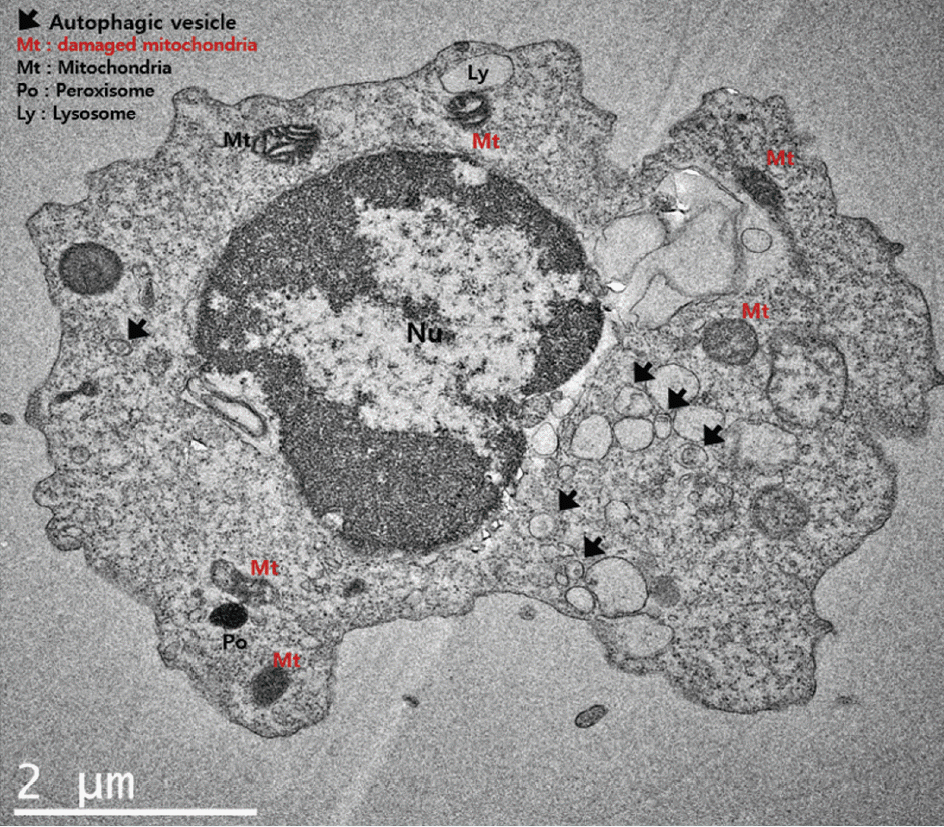
Fig. 2.
Gene ontology network of biological processes (A), molecular function (B), cellular components (c), and the protein-protein interaction network (d) of differentially expressed markers of autophagy and mitophagy associated with delayed cerebral ischemia following subarachnoid hemorrhage. p62 is also called SQSTM1. BNIP3L : BcL2 interacting protein 3 like, PINK1 : phosphatase and tensin homolog-induced kinase 1, SQSTM1 : sequestosome 1, dAPK : death-associated protein kinase.
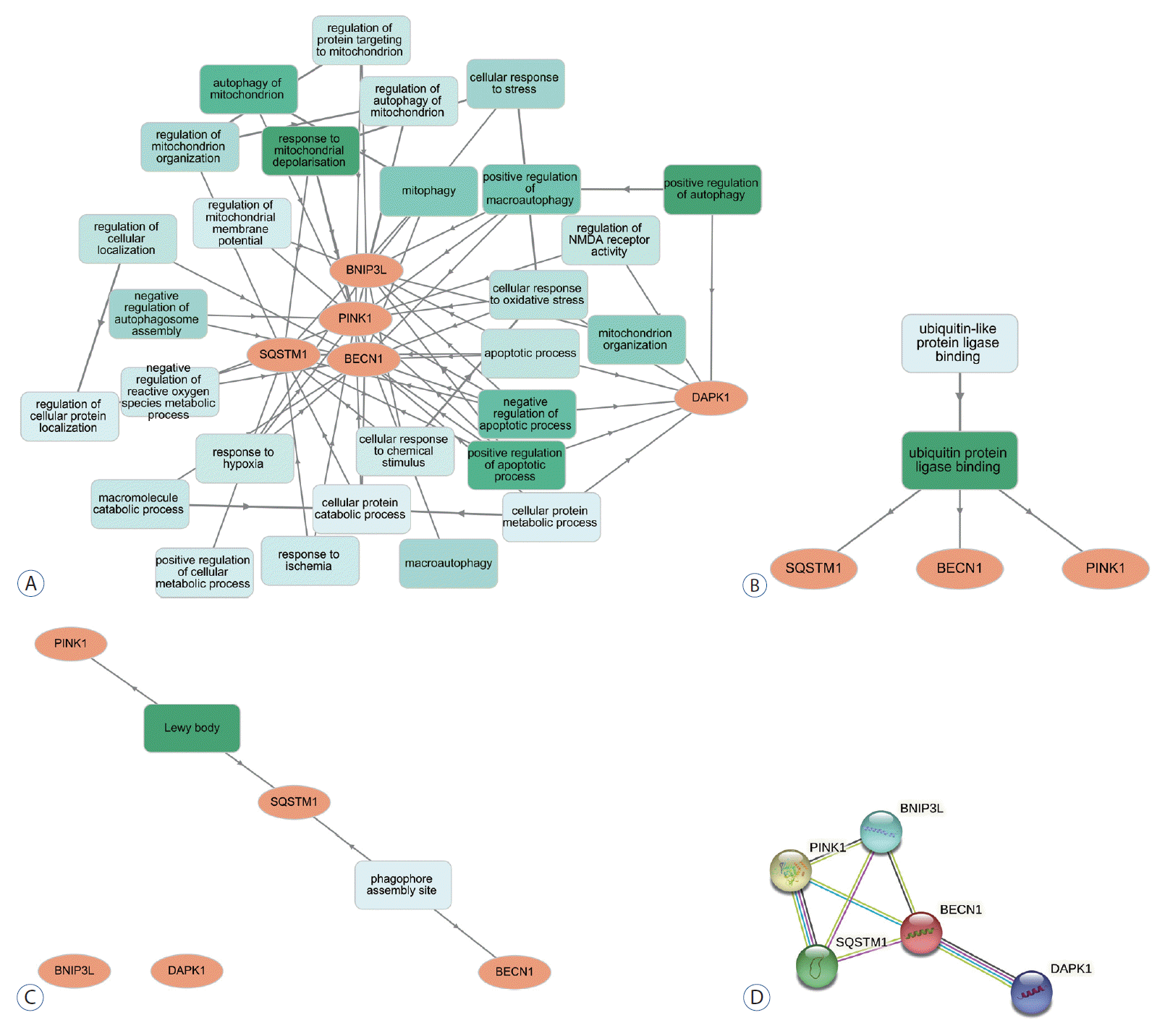
Fig. 3.
Kyoto Encyclopedia of Genes and Genomes pathway analysis of autophagy (A) and mitophagy (B). differentially expressed autophagy and mitophagy markers associated with delayed cerebral ischemia are marked in red. p62 is also designated sequestosome 1 (SQSTM1).
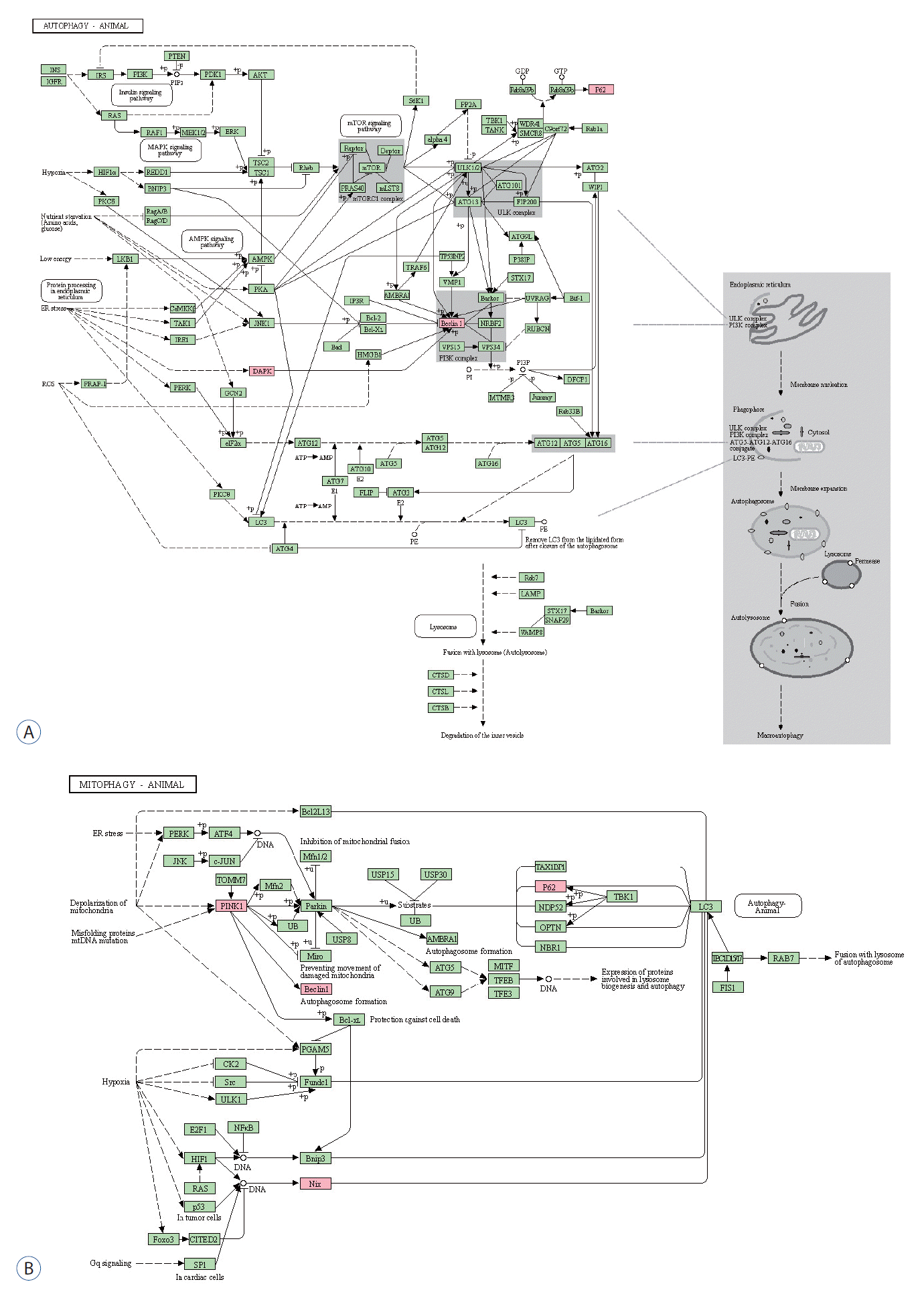
Table 1.
Gene ontology enrichment analysis of differentially expressed autophagy and mitophagy biomarkers in the cSF cells associated with delayed cerebral ischemia in patients with subarachnoid hemorrhage
| ID | GO term | p-value | Gene | |
|---|---|---|---|---|
| Biological process | ||||
| GO:0010508 | Positive regulation of autophagy | 6.40E-09 | BECN1, BNIP3L, DAPK1, PINK1 | |
| GO:0098780 | Response to mitochondrial depolarization | 7.50E-09 | BECN1, PINK1, SQSTM1* | |
| GO:0043065 | Positive regulation of apoptotic process | 3.36E-08 | BECN1, BNIP3L, DAPK1, PINK1, SQSTM1 | |
| GO:0000422 | Autophagy of mitochondrion | 7.07E-08 | BECN1, PINK1, SQSTM1 | |
| GO:0043066 | Negative regulation of apoptotic process | 1.68E-07 | BECN1, BNIP3L, DAPK1, PINK1, SQSTM1 | |
| GO:0016239 | Positive regulation of macroautophagy | 3.70E-07 | BECN1, BNIP3L, PINK1 | |
| GO:0000423 | Mitophagy | 7.67E-07 | BECN1, SQSTM1 | |
| GO:0007005 | Mitochondrion organization | 1.17E-06 | BECN1, BNIP3L, PINK1, SQSTM1 | |
| GO:1902902 | Negative regulation of autophagosome assembly | 3.99E-06 | BECN1, PINK1 | |
| GO:0033554 | Cellular response to stress | 4.36E-06 | BECN1, BNIP3L, DAPK1, PINK1, SQSTM1 | |
| GO:0016236 | Macroautophagy | 5.14E-06 | BECN1, PINK1, SQSTM1 | |
| GO:0010821 | Regulation of mitochondrion organization | 7.32E-06 | BNIP3L, PINK1, SQSTM1 | |
| GO:0034599 | Cellular response to oxidative stress | 1.65E-05 | BECN1, DAPK1, PINK1 | |
| GO:0006915 | Apoptotic process | 2.07E-05 | BECN1, BNIP3L, DAPK1, SQSTM1 | |
| GO:0060341 | Regulation of cellular localization | 2.07E-05 | BECN1, BNIP3L, PINK1, SQSTM1 | |
| GO:2000310 | Regulation of NMDA receptor activity | 3.21E-05 | DAPK1, PINK1 | |
| GO:0009057 | Macromolecule catabolic process | 3.63E-05 | BECN1, BNIP3L, PINK1, SQSTM1 | |
| GO:1903146 | Regulation of autophagy of mitochondrion | 4.39E-05 | BNIP3L, PINK1 | |
| GO:1903214 | Regulation of protein targeting to mitochondrion | 4.82E-05 | BNIP3L, PINK1 | |
| GO:0001666 | Responsetohypoxia | 4.91E-05 | BECN1, BNIP3L, PINK1 | |
| GO:0002931 | Response to ischemia | 5.99E-05 | PINK1, SQSTM1 | |
| GO:0070887 | Cellular response to chemical stimulus | 6.57E-05 | BECN1, BNIP3L, DAPK1, PINK1, SQSTM1 | |
| GO:2000378 | Negative regulation of reactive oxygen species metabolic process | 7.28E-05 | BECN1, PINK1 | |
| GO:0051881 | Regulation of mitochondrial membrane potential | 1.23E-04 | BNIP3L, PINK1 | |
| GO:0031325 | Positive regulation of cellular metabolic process | 1.25E-04 | BECN1, BNIP3L, DAPK1, PINK1, SQSTM1 | |
| GO:1903827 | Regulation of cellular protein localization | 1.86E-04 | BNIP3L, PINK1, SQSTM1 | |
| GO:0044267 | Cellular protein metabolic process | 2.35E-04 | BECN1, BNIP3L, DAPK1, PINK1, SQSTM1 | |
| GO:0044257 | Cellular protein catabolic process | 2.87E-04 | BNIP3L, PINK1, SQSTM1 | |
| Molecular function | ||||
| GO:0044389 | Ubiquitin-like protein ligase binding | 3.62E-05 | BECN1, PINK1, SQSTM1 | |
| GO:0031625 | Ubiquitin protein ligase binding | 3.02E-05 | BECN1, PINK1, SQSTM1 | |
| Cellular component | ||||
| GO:0097413 | Lewy body | 1.43E-06 | PINK1, SQSTM1 | |
| GO:0000407 | Phagophore assembly site | 2.53E-05 | BECN1, SQSTM1 | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download