INTRODUCTION
MATERIALS AND METHODS
Concept and design of a pituitary retractor
 | Fig. 1.Schematic illustration of pituitary retractor. A : Early descent of the diaphragma sellae (DS) into sella turcica may obscure the remaining tumor. B : With rigid support of pituitary retractor (asterisk), the DS can be elevated to reveal the concealed tumor. The remaining tumor is easily removed under direct visualization without the interruption of the ballooned DS. T : tumor. |
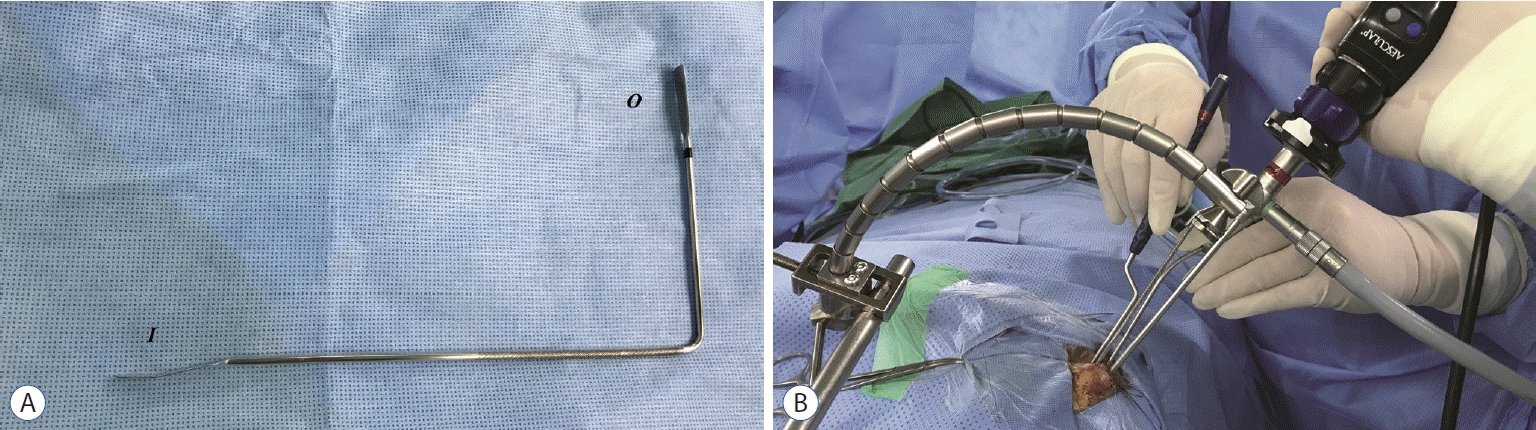 | Fig. 2.Application of new pituitary retractor. A : The metallic pituitary retractor is composed of three parts. Inner part (I) has a narrow and long spatula with slightly angled head to create a working space in the sellar cavity. The middle shaft is a slender rod with a right angle to avoid interference with instruments passing through the nostril. The outer part (O) is thin for fixation to the holding system. B : Pituitary retractor is introduced into left nostril and moved in the cephalic direction to create a working space below the pituitary retractor. The outer part is fixed with the Greenberg holder system (Codman, Dorchester, MA, USA). With fixation of the pituitary retractor, tumor resection is performed with bimanual maneuver. |
Surgical procedures
Patient population
Table 1.
Dx : diagnosis, W : width, H : height, Preop : preoperative, VA : visual acuity, VF : visual field, Intraop : intraoperative, DS : diaphragm sellae, CSF : cerebrospinal fluid, EOR : extent of resection, Cx : complication, M : male, NFA : nonfunctional pituitary adenoma, Hypo : hypopituitarism, GTR : grossly total removal, N/C : no change, Imp : improved, STR : subtotal removal, NL : normal, F : female, Det : deteriorated, LF : low flow, PRL : prolactinoma, Hyper : hyperpituitarism, HF : high flow, P. : pituitary, N/D : not described
RESULTS
Usefulness of pituitary retractor and extent of resection
Clinical outcome of patients
Illustrative case
Case No. 17
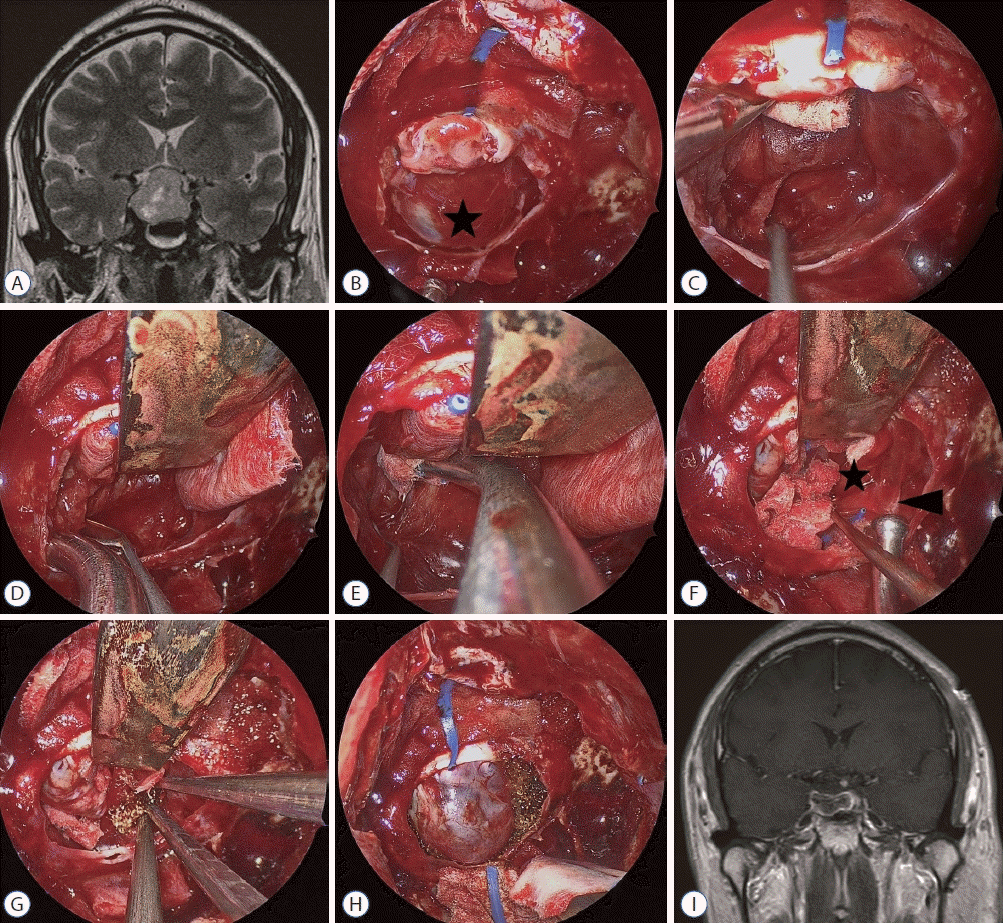 | Fig. 3.Case No. 17. A : Pituitary macroadenoma compresses optic nerve superiorly and extends laterally to the right superior cavernous compartment over the intracavernous ICA. B : The diaphragma sellae (DS, black asterisk) balloons out into the sella cavity during tumor removal. C : Tumor removal is proceeded using cottonoid patties to support the DS. However, the remaining tumor is not clearly identified due to the pooled blood and the limited maneuverability. D and E : A rigid pituitary retractor supports the DS stably, and the tumor around the right cavernous sinus is removed with a bimanual maneuver. F : Sharp dissection between the DS (black asterisk) and tumor capsule (black arrowhead) is performed under the DS. G : Hemostasis with surgicel is performed with bimanual maneuver under direct visualization. H : After the completion of tumor removal, the DS occupies the sella cavity where the rigid pituitary retractor is removed. I : On postoperative magnetic resonance image, the remaining tumor is not visible and the compressed optic chiasm is relieved. |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download