INTRODUCTION
Table 1.
FAILED JUNCTION WITH THE PRIMARY NEURAL TUBE
HYPOPLASIA OR ARREST OF SECONDARY NEURULATION ASSOCIATED WITH DISTURBED ACTIVITY OF CAUDAL MESENCHYMAL TISSUE
DUPLICATION OF THE CCM ASSOCIATED WITH DISTURBED ACTIVITY OF CAUDAL MESENCHYMAL TISSUE
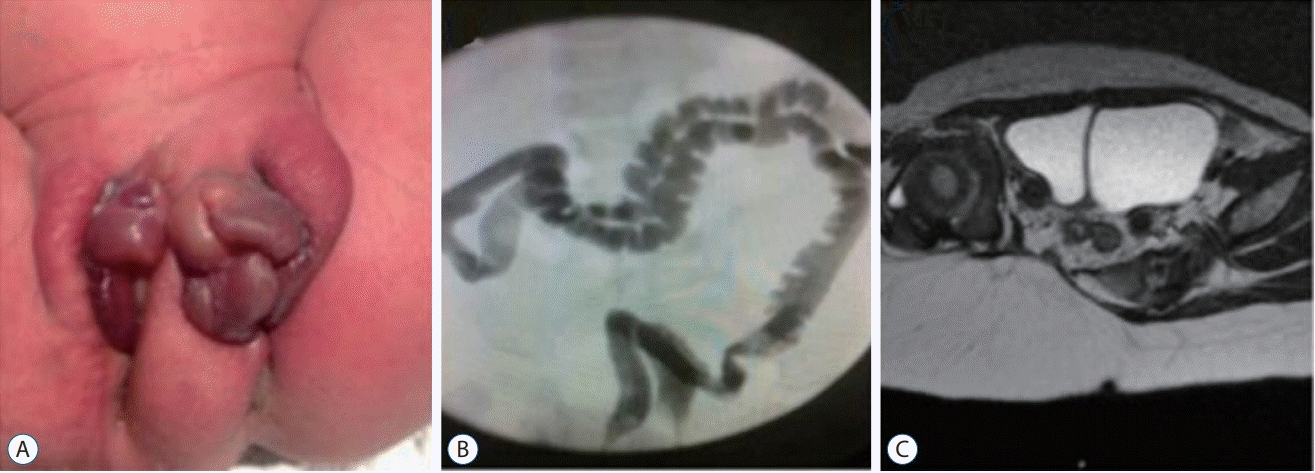 | Fig. 2. Common manifestations of caudal duplication syndrome. The proximal portion of duplicated organs is separated by a septum but not as two different independent organs. In contrast to two urethral, vaginal and anal orifices (A), duplicated colons run in parallel, sharing one septum between them (B). Similarly, duplicated bladders or uteri share a septum in the midline separating the two (C). Duplication occurs similar to the branching of a tree, followed by the formation of two sets of suborgans, such as the urethra, vagina and anus, until reaching the distal end. Reprinted from Harris et al. [24] with permission from Sage publications, Inc. |
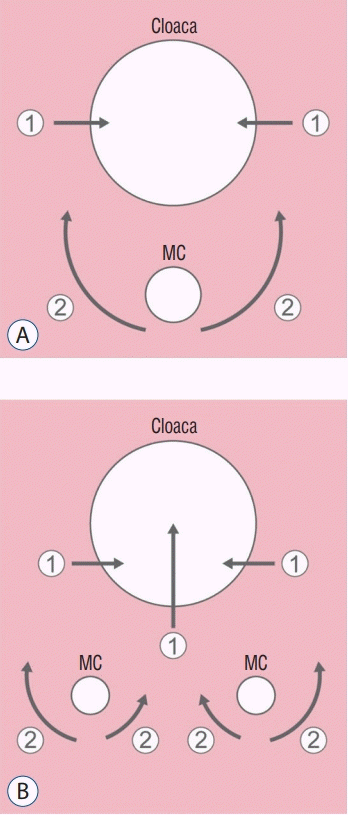 | Fig. 3.A schematic diagram of the hindgut including cloaca and medullary cord during the formation of the urogenital sinus and anorectal canal in the normal state (A) and in caudal duplication syndrome (CDupS) (B). The upper large circle indicates the cloaca, and the lower small circle(s) indicates the medullary cord. The arrow indicates the movement of mesenchymal cells, whereas the length of the arrow indicates the power of the movement. Arrows numbered ① indicate the formation of the septum (normally urorectal septum, additional midline septum in CDupS) in the cloaca by the mesenchymal push from both lateral walls in normal development and from the posterior wall as well in CDupS. Arrows numbered ② indicate the ventral push of the caudal mesenchyme. Duplicated medullary cords give rise to an aberrant insertion of mesenchymal tissue between the two medullary cords at the midline (B), which causes abnormal midline septation of the cloaca (or hindgut). Note that the ventral push of the mesenchyme around the lateral sides of the medullary cords in CDupS (B) is weaker than that in the normal state (A) due to the leakage of mesenchymal growth power through the midline. Weak ventral push along the lateral sides of the cloaca makes the urorectal septum locate more posteriorly than in the normal position, as in caudal agenesis, which may later result in an imperforate anus. Reprinted from Yang et al. [69] with permission from Springer Nature. MC : medullary cord. |
FAILED INGRESSION OF THE PRIMITIVE STREAK TO THE CCM
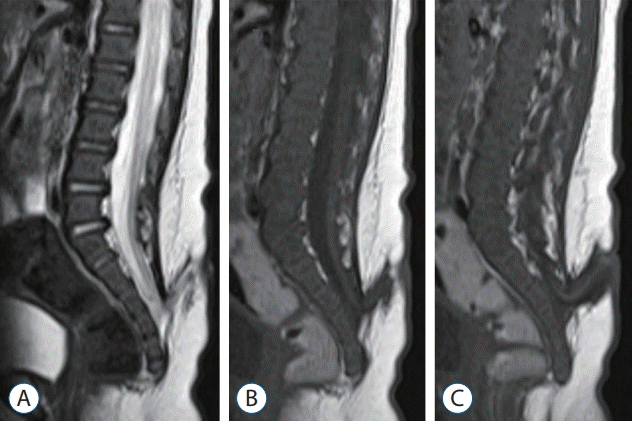 | Fig. 4.A 2-month-old girl who was born with a sacral myelomeningocele (MMC) and underwent a delayed operation : T2 (A) and T1 (B and C) magnetic resonance imaging sections show a typical feature of an MMC, but the bony defect is low, below S3. The distal spinal cord turns to the cranial side. |
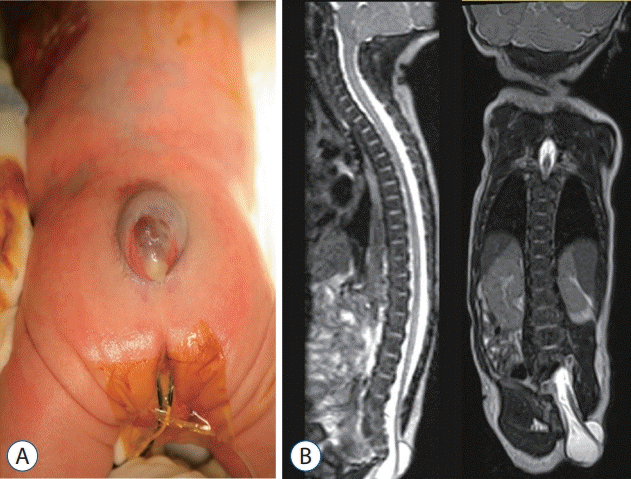 | Fig. 5.A 1-day-old girl with a fetal diagnosis of a myelomeningocele. A : A gross photo showing the cystic sac with a skin defect at the sacrococcygeal level. B : T2 sagittal and coronal sections of magnetic resonance imaging showing the low-lying cord protruding through the fascia defect at the S2 level and below, going straight to the caudal side and attached to the skin defect area. |
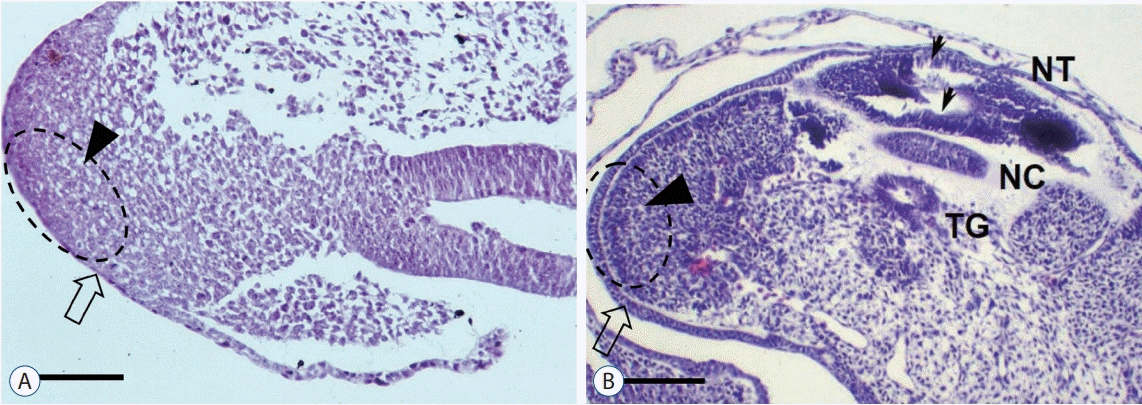 | Fig. 6.The surface ectoderm (open arrow) and the caudal cell mass (arrowhead) are continuous in a Hamburger and Hamilton (H-H) stage 16 chick embryo (A; H&E, scale bar=100 μm). At H-H stage 20, the two layers are clearly divided (B; H&E, scale bar=100 μm). Small arrows indicate multiple vacuoles in the secondary neural tube. Reprinted from Kim et al. [27] by permission of the Congress of Neurological Surgeons. NT : neural tube, NC : notochord, TG : tailgut. |
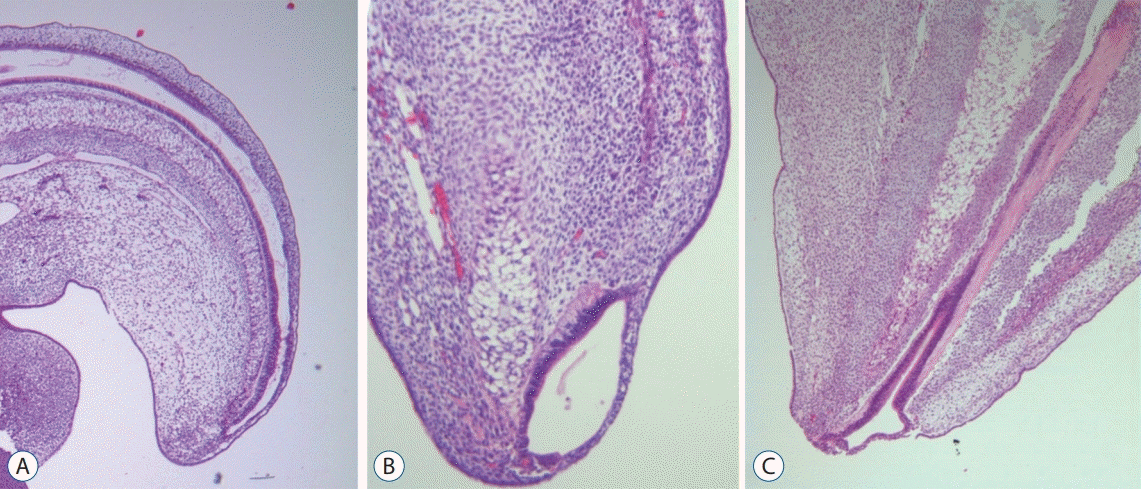 | Fig. 7.Serial images of chick embryos showing the central canal of the medullary cord (A; H&E, ×40), a terminal balloon seen as a focal dilatation of the
distal end of the central canal (B; H&E, ×200), and the shrinkage of the terminal balloon (C; H&E, ×200). Modified from Lee et al. [32] with reprint permission
from Springer Nature. |
FOCAL LIMITED DORSAL NEURO-CUTANEOUS NONDISJUNCTION
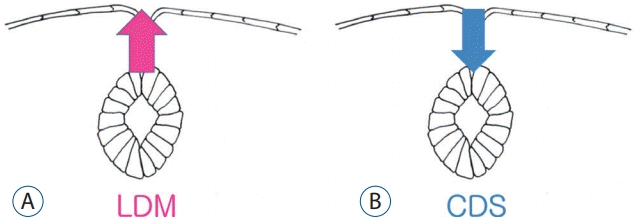 | Fig. 8.A schematic drawing showing the pathoembryogenesis of limited dorsal myeloschisis (LDM) compared with congenital dermal sinus (CDS). The common basic error is a failure of normal neurocutaneous disjunction during primary neurulation. If the neural tissue is pulled up to the skin side, it results in LDM (A), whereas if the cutaneous tissue is pulled down to the neural side, it leads to CDS (B). |
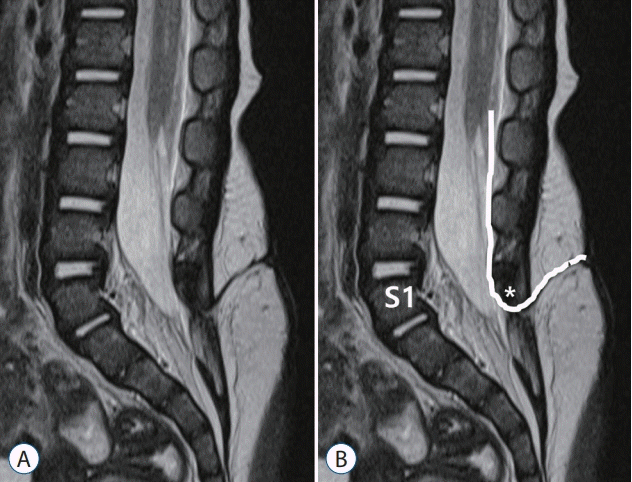 | Fig. 9.A drawing showing the method to determine the spinal level of limited dorsal myeloschisis (LDM). A : T2 sagittal image. B : A method of counting the level of LDM. An LDM stalk traverses through the S1–2 interspinous ligament. The spinal level of this LDM was regarded as S1. Asterisk indicate dysplastic small spinous process of S1. |
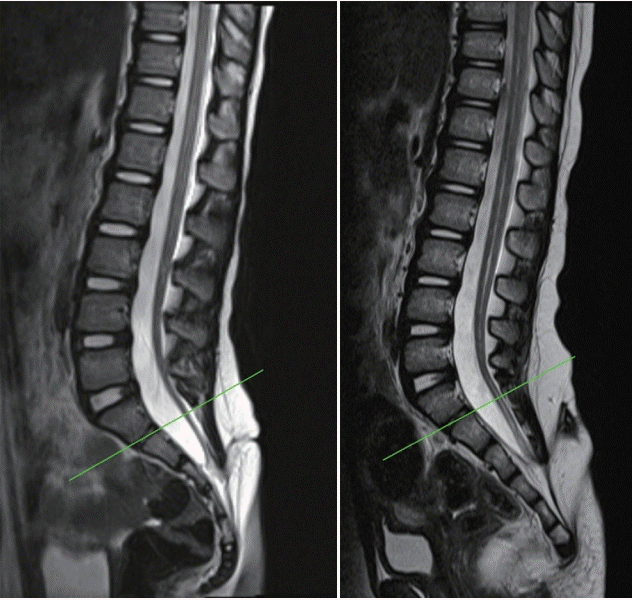 | Fig. 10.Cases with low-lying limited dorsal myeloschisis (LDM). The spinal levels of both LDM cases are S3. The LDM stalks were attached to the low-lying coni. Green line indicate level of S1–2 junction (the LDM tracts passing though the interspinous spaces below this line seem attached to the spinal cord below S1–2 junction which was known to be formed by secondary neurulation only, not by primary neurulation). |
NEURO-MESENCHYMAL ADHESION
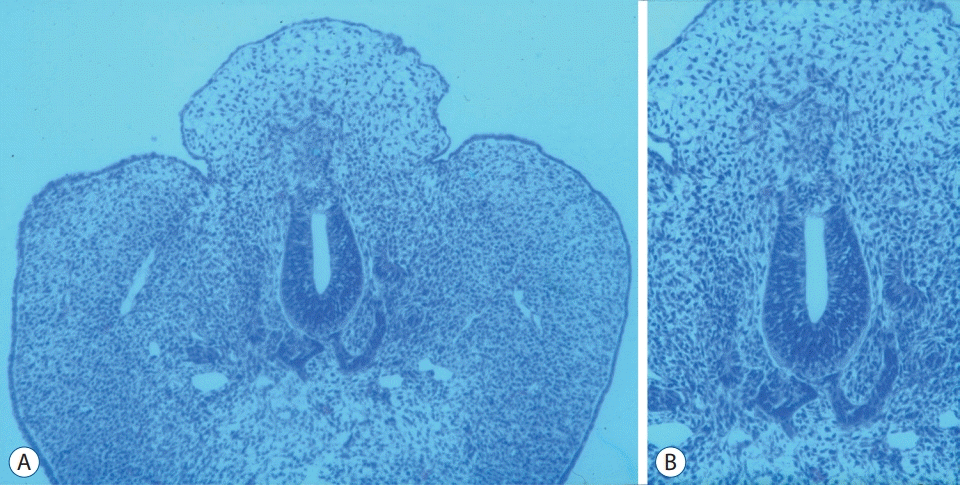 | Fig. 11.A and B : A chick embryo showing histological findings suggestive of lumbosacral lipomatous malformation after the incision of the unilateral neural fold : a back hump making a sharp angle with surrounding normal skin, the blending of neuroepithelial and mesenchymal tissues through an indistinct basement membrane and an abnormal shape of the notochord (A : H&E, ×100; B : H&E, ×200). Reprinted from Li et al. [36] with permission from S. Karger AG. Basel. |
Transitional type
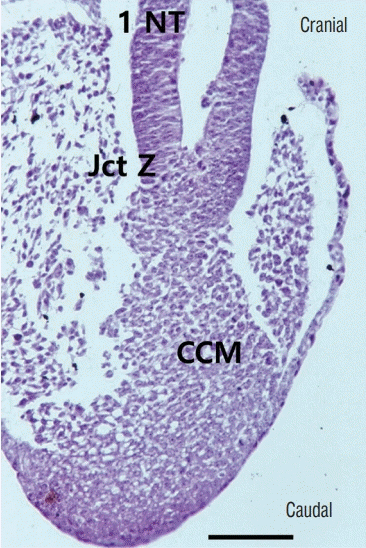 | Fig. 12.The junction between the primary neural tube and the CCM in a chick embryo of Hamburger and Hamilton stage 16 (a sagittal section, slightly off the midline). The CCM makes the medullary cord by the process of secondary neurulation from Hensen’s node to the caudal side of the embryo. H&E, scale bar=100 μm. 1 NT : primary neural tube, Jct Z : junctional zone between the primary and secondary neural tubes, CCM : caudal cell mass. |
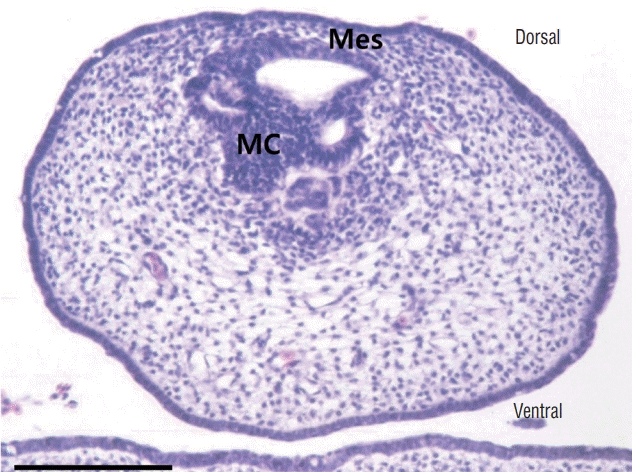 | Fig. 13.A transverse section of a medullary cord in a chick embryo of Hamburger and Hamilton stage 30. The mesenchymal tissue between the medullary cord and skin is visible. There are still multiple vacuoles in the medullary cord. If mesenchymal tissue adheres to the dorsal aspect of the medullary cord during the process of secondary neurulation, lumbosacral lipomatous malformation of the transitional type will occur. H&E, scale bar=50 μm. Mes : mesenchymal tissue, MC : medullary cord. |
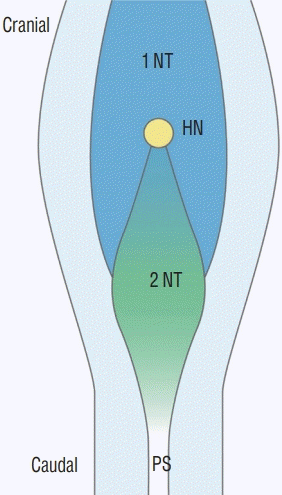 | Fig. 14.A schematic drawing of Hensen’s node area in a chick embryo of Hamburger and Hamilton stage 8. The caudal part of Hensen’s node in the midline is the future secondary neural tube where the secondary neurulation progresses to the caudal side. The neural plates of the caudal primary neural tube are located at the lateral sides. If the caudal part of Hensen’s node (and the cranial part of the 'node-streak border' or ’rhomboidal area') has neuro-mesenchymal adhesion, the caudal end of primary neurulation is disturbed, and the fat is attached to the dorsal spinal cord up beyond the normal dorsocaudal end of the primary neural tube, which is known as the junction of S1–2 spinal cord segments. Modified from Shimokita and Takahashi [58] with permission from Japanese Society of Developmental Biologists. 1 NT : primary neural tube, HN : Hensen’s node, 2 NT : secondary neural tube, PS : primitive streak. |
Caudal type
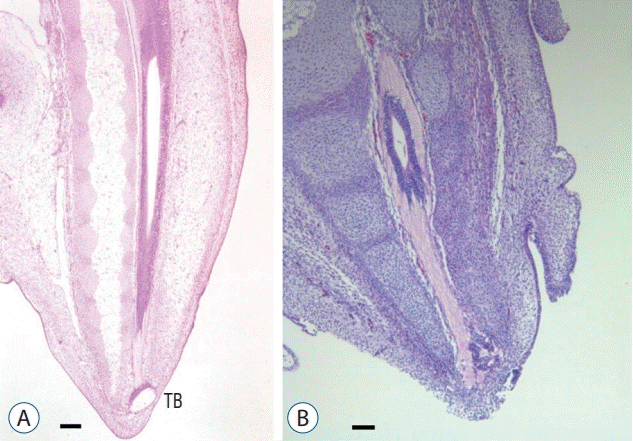 | Fig. 15.Hamburger and Hamilton stage 30–35 chick embryos show the fully formed medullary cord with a terminal balloon (terminal vesicle) (A; H&E, scale bar=100 μm) and then regression of the balloon (B; H&E, scale bar=100 μm). TB : terminal balloon. |
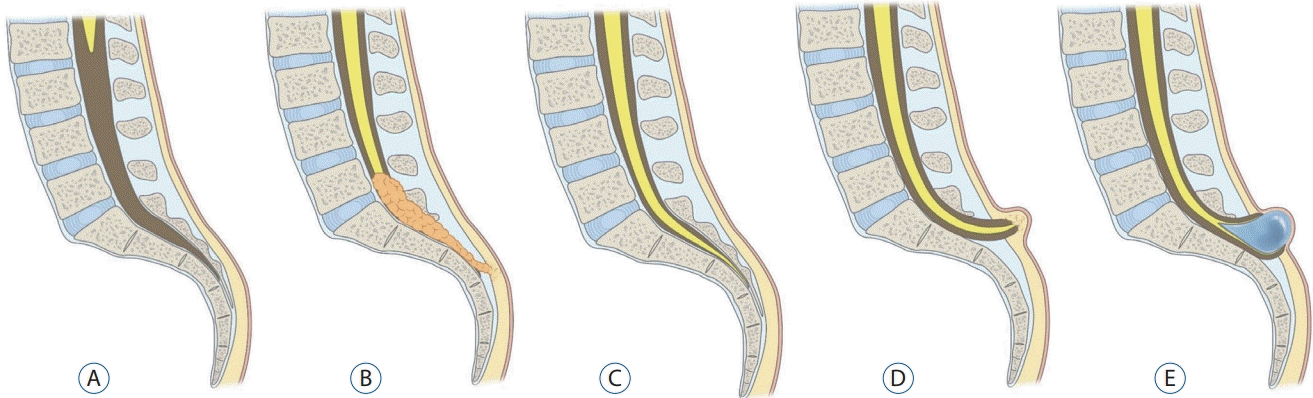 | Fig. 16.A : A normal caudal spinal cord. B : If the site of neuro-mesenchymal adhesion at the caudal end of the medullary cord was pulled into the spinal canal through the sacral hiatus, a lumbosacral lipomatous malformation of the caudal type is formed. C : However, when abnormal neuro-mesenchymal adhesion occurs but there is no traction of mesenchymal tissue into the neural side, it may result in a typical retained medullary cord. D and E : If the spinal cord is distracted to the extraspinal side, a terminal myelocele (if the terminal balloon collapses, D) or terminal myelocystocele (if the terminal balloon persists, E) is formed. |
Filar type
REGRESSION FAILURE SPECTRUM OF THE MEDULLARY CORD
Filar lesions
RMC
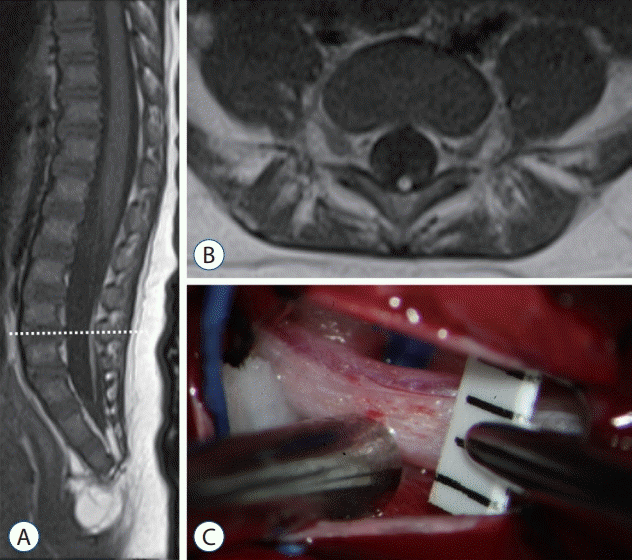
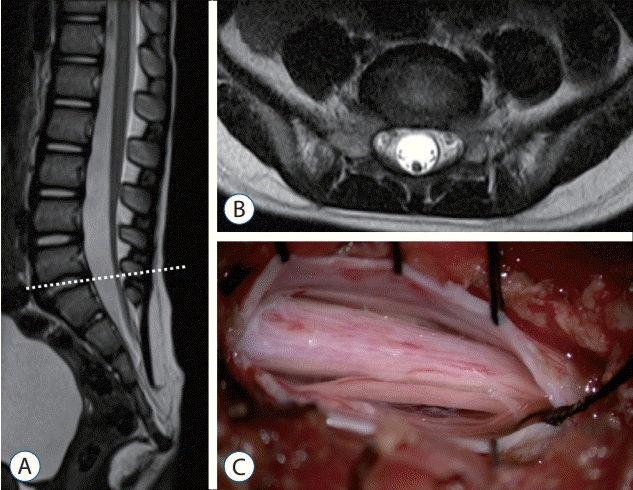 | Fig. 19.Magnetic resonance imaging and an intraoperative photograph in a retained medullary cord patient. A : A T2 sagittal image. A cord-like structure extending to the sacral area. B : T2 axial images at the L5–S1 level (dotted line in A). C : Left L5 partial hemilaminectomy shows a thick, pia-covered medullary cord passing through. Reprinted from Kim et al. [28] with permission from the Korean Neurosurgical Society. |
TMC
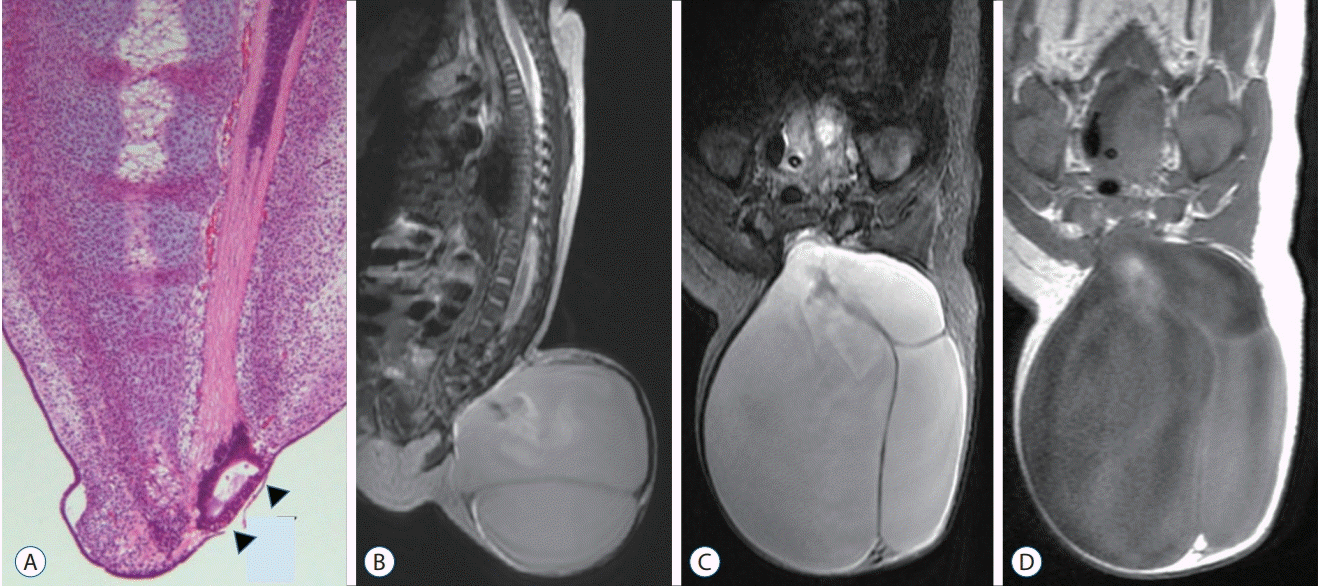
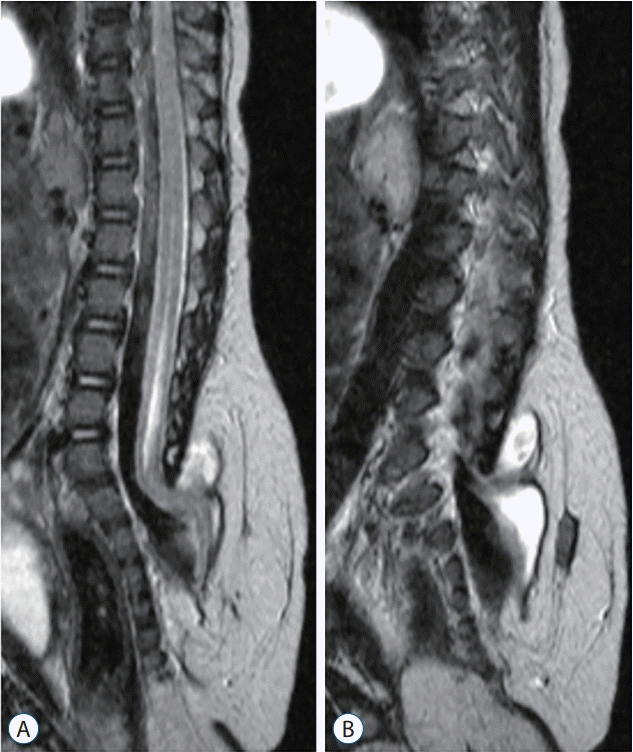 | Fig. 20.Sagittal magnetic resonance imaging images of a case with terminal myelocele show the herniated spinal cord through laminar and fascial defects. Trumpet-like flaring is not seen. Only an enlarged subarachnoid space and herniated spinal cord and roots are observed. A : T2 sagittal image, midline plane. B : T2 sagittal image, paramedian plane where the herniated spinal cord is best seen. Reprinted from Lee et al. [32] by permission from Springer Nature. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download