INTRODUCTION
Split cord malformation (SCM), a congenital neural tube defect, presents with longitudinally separated functional hemicords. Pang et al. [
16] classified SCM into two types. In type I SCM (diastematomyelia), the hemicords are divided by a bony sagittal septum within double dural sacs. In type II SCM (diplomyelia), the hemicords are divided by a thin sagittal fibrous septum within a single dural sac [
16]. However, some authors have reported a few cases demonstrating characteristics of both type I and type II SCM, that is, mixed, intermediate, or composite type SCM [
2,
11,
18]. Recently, Meena et al. [
15] suggested type 1.5 SCM to define these unusual cases. In this report, we present two cases of type 1.5 SCM and review the relevant literature. We also discuss the previously hypothesized pathogenesis and propose a new possible pathogenetic theory.
Go to :

DISCUSSION
We presented two different cases of type 1.5 SCM in this report. Due to the rarity of type 1.5 SCM, these two cases are rather valuable. Furthermore, we reviewed the pertinent literature to cover detailed information of published reports, which could help neurosurgeons obtain more knowledge regarding type 1.5 SCM.
SCM is a congenital abnormality, within the spectrum of neural tube defects, in which a bony or fibrous septum longitudinally divides the spinal cord into two parts. Due to distinct conceptions, previous authors named SCM with confusing terminology. Diastematomyelia was adopted by Hertwig [
10] and supported by some other authors [
12,
14], while Bruce et al. [
4] proposed diastematomyelia for the hemicords with a midline bony spur and diplomyelia for the hemicords without the spur. Other authors, however, preferred diplomyelia regardless of the septal components [
9,
13,
17].
In 1992, Pang et al. [
16] proposed a novel nomenclature that classified SCM into two types : type I SCM (formerly diastematomyelia), presenting with hemicords within double dural sacs split by a bony sagittal septum and type II SCM (formerly diplomyelia), in which hemicords lie in an intact single dural sac with a thin midline sagittal fibrous septum. They also postulated a unified theory to elucidate the pathogenesis of SCM during embryogenesis [
16]. According to the unified theory, both types result from a common ontogenetic error [
16]. The meninx primitiva appears over the ventral neural tube and migrates dorsally to wrap it, forming the dural sac. If an abnormal midline fistula of the primitive dura mater forms simultaneously with the normal primitive neurenteric canal (at approximately 21 days’ gestational age), the mesenchyme will condense around the fistula, form an endomesenchymal tract, and recruit the meninx primitiva into the tract (around 30 days’ gestational age). It is this meninx primitiva that differentiates into the future dura mater and bony septum, and type I SCM develops. If the endomesenchymal tract appears earlier than day 21, the mesenchyme will not collect the meninx primitiva. This results in a thin fibrous septum distinguishable from normal dura mater between the hemicords or type II SCM. Pang et al. [
16] believed that the unified theory was validated by the fact that all 39 patients with SCM in their series did not manifest combined features of types I and II.
However, some authors have reported a few cases with characteristics of both types I and II SCM, meaning that the lesion incorporated the hemicords within a single dural sac and an extradural partial bony septum [
1,
3,
5,
7,
8,
15,
18,
19,
21]. We reviewed the literature, and 13 pertinent cases are listed in
Table 1.
Table 1.
|
Study |
Age (years)/sex |
Clinical presentation |
Radiological presentation |
Intraoperative presentation |
|
Chandra et al. [5] (1999) |
9/F |
Right-sided sprengel shoulder deformity |
A dorsal bony septum at L2–3 |
Thick filum terminale |
|
Lumbosacral hair |
Split cord beginning at L2 |
No fibrous extension |
|
Tethered cord at L4 |
|
Low conus at L5 |
|
Erşahin [7] (2000) |
14 months/M |
Lumbar hypertrichosis |
A dorsal bony septum at L3 |
Tight filum terminale |
|
Widened spinal canal |
Fibrous bands and dorsal paramedian roots between the dorsal surface of hemicords and the dorsal dura |
|
Bifid laminae at L5–S1 |
No fibrous extension |
|
Basak et al. [3] (2002) |
3/F |
Hair-covered mass of the upper dorsal spine |
A dorsal bony septum at C5–6 |
No fibrous extension |
|
Split cord at C3–T1 |
|
Syrinx cavities at C3–T1and T3–T7 |
|
Akay et al. [1] (2002) |
7/F |
Lumbar hair |
A dorsal bony septum at L4 |
Rootlets coming out from the medial aspect of the hemicords |
|
Low conus at L4 |
No fibrous extension |
|
Akay et al. [1] (2002) |
2/F |
A sacral rigid mass lesion |
A dorsal bony septum at S1 |
Rootlets coming out from the dorsal medial aspect of the hemicords |
|
A bifid lamina at S1 |
Thick filum terminale |
|
Conus medullaris at coccyx |
Fibrous ligaments lying on the dorsal dura |
|
No fibrous extension |
|
Van Aalst et al. [21] (2005) |
15/M |
Walking difficulties |
A dorsal bony septum at L3–L4 |
Arachnoidal adhesions between the hemicords |
|
Pain in the lower extremities, scoliosis |
Fibrous extension to the ventral dura |
|
Singh et al. [19] (2011) |
1/F |
Hair over the middorsal spine with hyperpigmentation |
A bony septum at T5–T9 |
Hemicords with separate dura sacs at T2–L1 |
|
A ventral bony septum at T11 |
Hemicords with a single dura sac at T11 |
|
Split cord at T2–L1 |
Thick filum terminale |
|
Low conus at L3–4 |
No fibrous extension |
|
Salunke et al. [18] (2011) |
7/M |
A lipomatous swelling |
A ventral bony septum at lower dorsal level |
The paramedian nerve roots attaching to one another |
|
Lumbar hypertrichosis |
Bifid laminae |
Sinus tract extending extradurally |
|
Weakness of the left lower limb |
No fibrous extension |
|
Salunke et al. [18] (2011) |
1/M |
A small midline swelling at the lower back |
A lumbosacral dorsal bony septum |
Fibrous extension to the ventral dura |
|
Low conus to the sacrum |
|
Garg et al. [8] (2015) |
10 months/F |
Lumbar midline hair |
A ventral bony septum at L2–L3 |
Thick filum terminale |
|
Lower limbs moving less |
Lumbosacral spinal dysraphism |
No fibrous extension |
|
Scoliosis |
Low conus at L3 |
|
Meena et al. [15] (2020) |
11/M |
Low back pain |
A ventral bony septum at T12–L1 |
Thick filum terminale |
|
Split cord from T11 to the cord ending |
No fibrous extension |
|
Low conus at L3–L4 |
|
Hypoplastic T10–L2 vertebral body |
|
Present case 1 |
52/F |
A lumbosacral dermal sinus with central hyperpigmentation and tenderness |
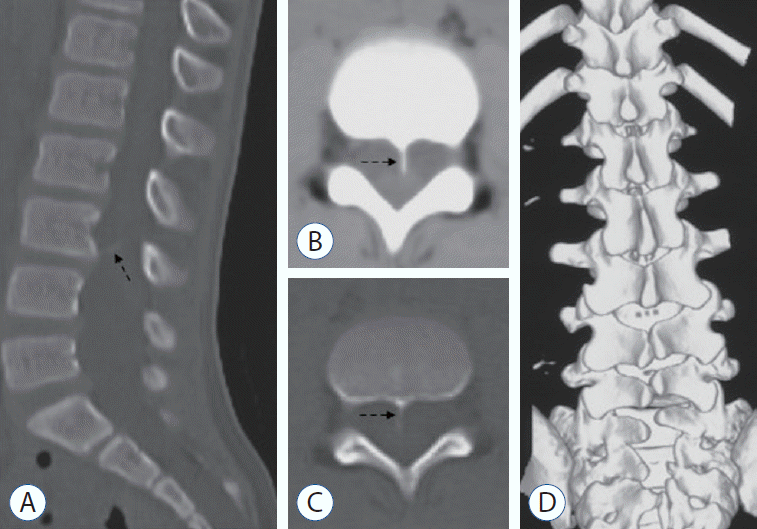
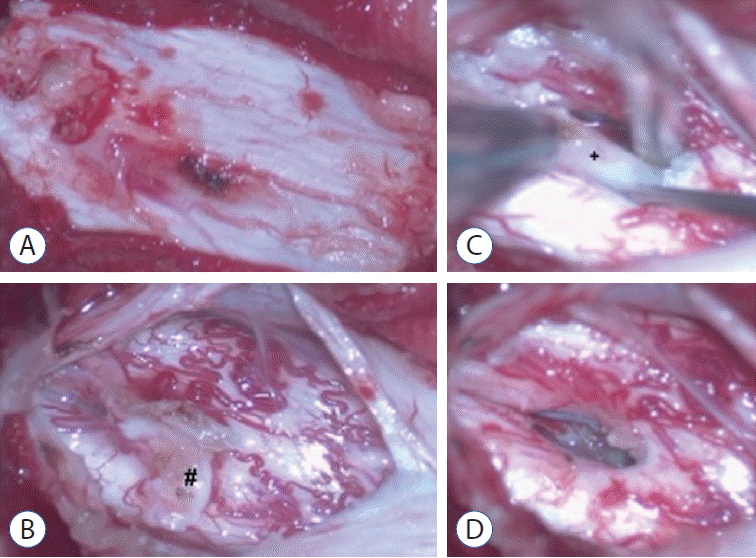
A dorsal bony septum at L5 |
Sinus tract communicated with intradural space |
|
Pain on the dorsal side of both lower limbs |
Low conus at S1 |
Thick and tight filum terminale |
|
Numbness of the left foot |
Lumbosacral spina bifida |
No fibrous extension |
|
Present case 2 |
9/M |
Asymmetric shoulders |
A ventral bony septum |
Filum terminale lipoma |
|
Scoliosis |
Low conus at L5 |
Fibrous extension to the dorsal dura |
|
A lumbosacral wrinkled cutaneous pit |
Butterfly vertebra at T4 |
|
Urine suppression |
Lumbosacral spina bifida |

There is a lack of consensus on the terminology for this combined SCM, whose terminology includes mixed SCM [
18], intermediate SCM [
11], and composite SCM [
16]. Recently, Meena et al. [
15] proposed type 1.5 SCM to define this unusual variation. Based on the location of the bony septum, they classified type 1.5 SCM into two subtypes : type 1.5a SCM, with the vertebral plate arising, and type 1.5b SCM, with the vertebral body arising [
15]. We believe that this simplified terminology adequately describes the characteristics of this unusual SCM. Referring to this method, we classified our case 1 as type 1.5a SCM and case 2 as type 1.5b SCM. We hope that this terminology will be adopted by more authors and applied in future studies to unify the nomenclature.
Different authors have held various views to explain the pathogenesis of type 1.5 SCM. Vaishya and Kumarjain [
20] supported the unified theory and considered that all variants of SCM shared a common pathogenesis in which the proportion of the meninx primitiva was the only influencing factor determining the SCM type : the more meninx primitiva cells present in the endomesenchymal tract, the more complete is bony septum and dura formation. Chandra et al. [
5] reported a case of dorsal bony septum originating from the vertebral arch. They questioned the unified theory in which the bony septum should develop from the vertebral body where the migration of the meninx primitiva begins [
16], and hypothesized that the meninx primitiva first migrates along the lateral side of the hemicords, accumulates dorsally, and returns along the endomesenchymal tract dorsoventrally, then forms a dorsal bony septum [
5]. In the unified theory, the bony septum and hypertrophic fusion of adjacent laminae should co-exist at the same level in type I SCM, because the meninx primitiva in the endomesenchymal tract could simultaneously induce both lesions [
16]. Salunke et al. [
18] reported two cases of type I SCM with normal laminae; hence, they doubted the value of the meninx primitiva in forming the bony septum and pointed out a possible role of the pluripotent mesenchyme. They also surmised that the regression of the endomesenchymal tract might cause type 1.5 SCM [
18].
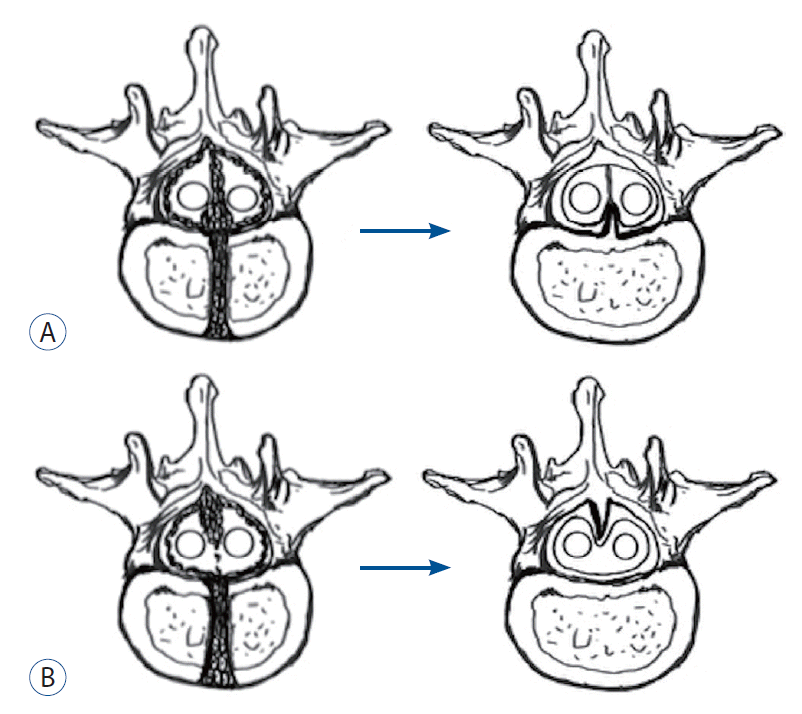
To explain the formation of all subtypes of type 1.5 SCM described in previous reports and our cases, we postulate an associative pathogenesis of type 1.5 SCM, consisting of uneven distribution of meninx primitiva and regression of the bony septum. In case 1, we intraoperatively found a dorsal bony septum at the L5 level and fused laminae of L4 and L5. Case 2, however, had normal laminae opposite to the ventral bony septum. We conjecture that uneven distribution of the meninx primitiva between the hemicords might cause less cells of the meninx primitiva to mix with the neural arch cells, which weakens the sclerosing effect [
2], resulting in normal laminae without hypertrophic fusion. This uneven distribution could also explain Salunke et al.’s cases [
18]. Case 2 had a fibrous extension from the bony septum to the opposite dura, but case 1 did not. We hypothesize the regression of the bony septum as a possible mechanism for this left fibrous extension because some studies have validated that regression plays a key role in neurulation and neural tube development [
6,
22]. In other words, type 1.5 SCM might be derived from type I SCM. In type I SCM, with uneven distribution, less meninx primitiva forms a less stable part of the bony septum. With some unknown factors, this unsubstantial part might regress, leaving a half bony septum. In case 2, we consider that relatively fewer cells of the meninx primitiva near the neural arch formed neither hypertrophic laminae nor a solid dorsal bony septum. Some factors might drive the dorsal part to regress into a fibrous extension (
Fig. 6A). In case 1, it is possible that few meninx primitiva cells exist around the vertebral body, resulting in a remnant dorsal bony septum without any ventral fibrous extension (
Fig. 6B), which could also explain the case presented by Chandra et al. [
5]. Therefore, we postulate that the presence of a fibrous extension might depend on the amount of condensing meninx primitiva. Considering that type 1.5 SCM is extremely rare, we regard regression to be accidental. Pang et al. [
16] reported that type II SCM was more frequent than type I SCM (around 60% and 40%, respectively). Hence, we believe that type II SCM in which only fibrous septa exist should not regress from type I SCM. The pathogenesis of type I and II SCM defined in the unified theory should be conceivable, and our associative theory is only responsible for type 1.5 SCM. To explicitly describe the true mechanisms, an experimental model of embryogenesis may be necessary in the future.
 | Fig. 6.A relatively lower proportion of meninx primitiva near the neural arch regressing into a fibrous extension from the ventral bony septum in case 2 (A). Few meninx primitiva cells existing around the vertebral body, resulting in a regressed dorsal bony septum without any ventral fibrous extension in case 1 (B). 
|
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download