INTRODUCTION
MATERIALS AND METHODS
Patient demographics
Operative technique
Assessment of complications
Table 1.
Prolonged C-reactive protein (CRP) normalization
Operation time, EBL, and transient psoas paresis according to the learning curve
Statistical analysis
RESULTS
Table 2.
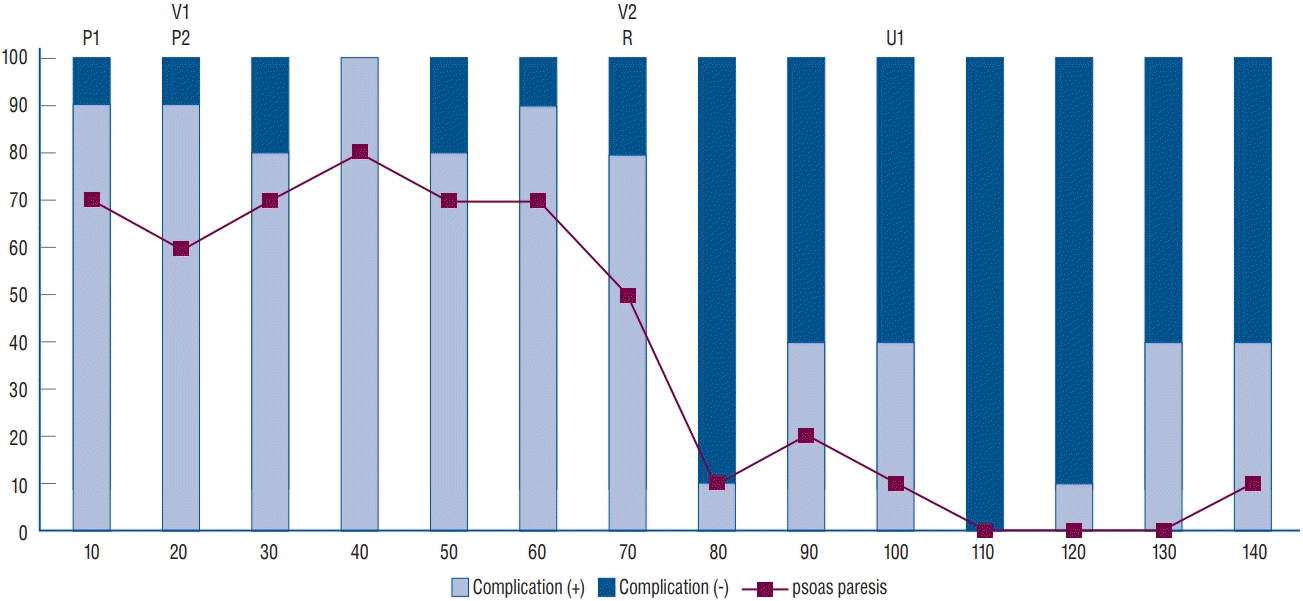 | Fig. 1.Changes in complications with case accumulation. The proportion of complications was relatively high in the initial 70 patients, and psoas paresis was the most common complication. The incidence of complications significantly decreased in the 70 patients in the late stage. P1 and P2 : two cases of peritoneal laceration, V1 and V2 : two cases of vascular injury, R1 and R2 : two cases of reoperation (R1 : cage repositioning, R2 : vertebral body fracture), U1 : one case of injury to the ureter. |
Table 5.
CRP : C-reactive protein, DM : diabetes mellitus, BMI : body mass index, ASA : American Society of Anesthesiologists Classification, Post. Decomp : posterior decompressive laminectomy, Post. Extension : level of posterior fixation in addition to the oblique lateral interbody fusion level, EBL : estimated blood loss, previous op : previous operation, M : male, F : female
Learning curve
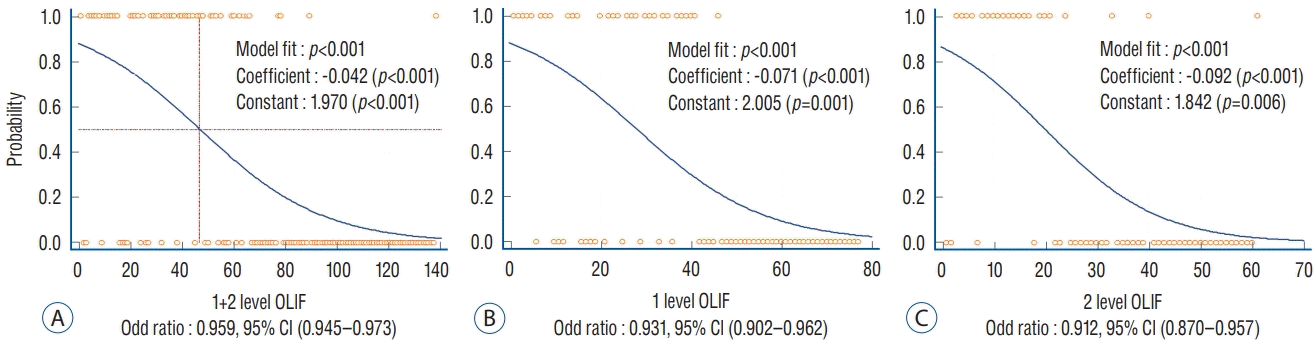 | Fig. 2.Binomial logistic regression curve for the change in psoas paresis with case accumulation. A : 1+2-level OLIF. B : 1-level OLIF. C : 2-level OLIF. In both 1- and 2-level OLIF, psoas paresis decreased significantly according to the accumulation of cases. OLIF : oblique lateral interbody fusion, CI : confidence interval. |
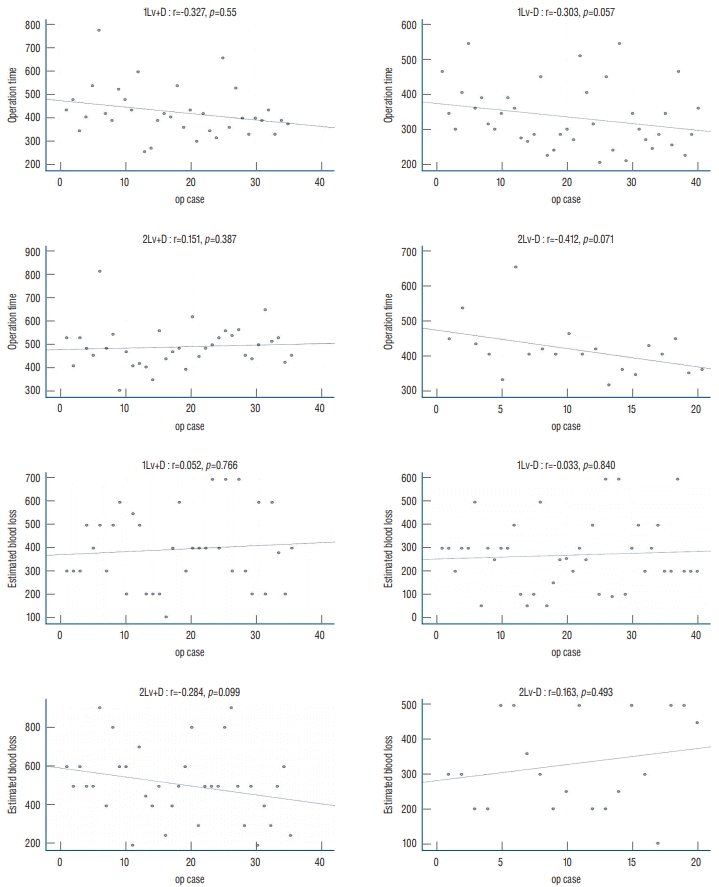 | Fig. 3.Scatter plot of the change in operation time (upper 4 graphs) and estimated blood loss (lower 4 graphs) according to consecutive cases in four groups. R : Spearman’s rank correlation coefficient, 1Lv : 1-level oblique lateral interbody fusion (OLIF), 2Lv : 2-level OLIF, +D : with decompressive laminectomy, -D : indirect decompression. |
OLIF-specific complication cases
Cage malposition
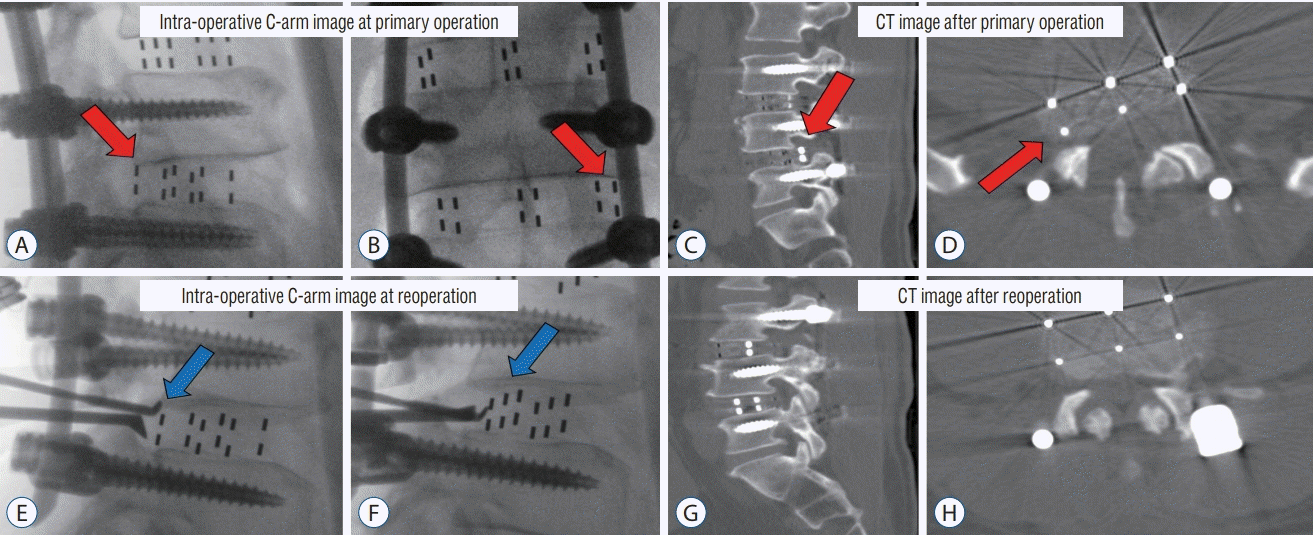 | Fig. 4.A-D : Case of cage malposition (red arrow) requiring revision surgery. Intraoperative C-arm image at the primary operation. A : The posterior margin of the cage is located inside the posterior margin of the vertebral body. B : The right lateral margin of the cage is located on the interpedicle line. CT image after the primary operation. C and D : Cage is located in the right neural foramen. E and F : Intraoperative cage reposition (blue arrow) using C-arm image at reoperation. After cephalic retraction of the root (E), the impactor is placed on the cage and repositioned under the C-arm guide (F). G and H : CT image after reoperation. Cage repositioning confirmed. CT : computed tomography. |
Vertebral body fracture
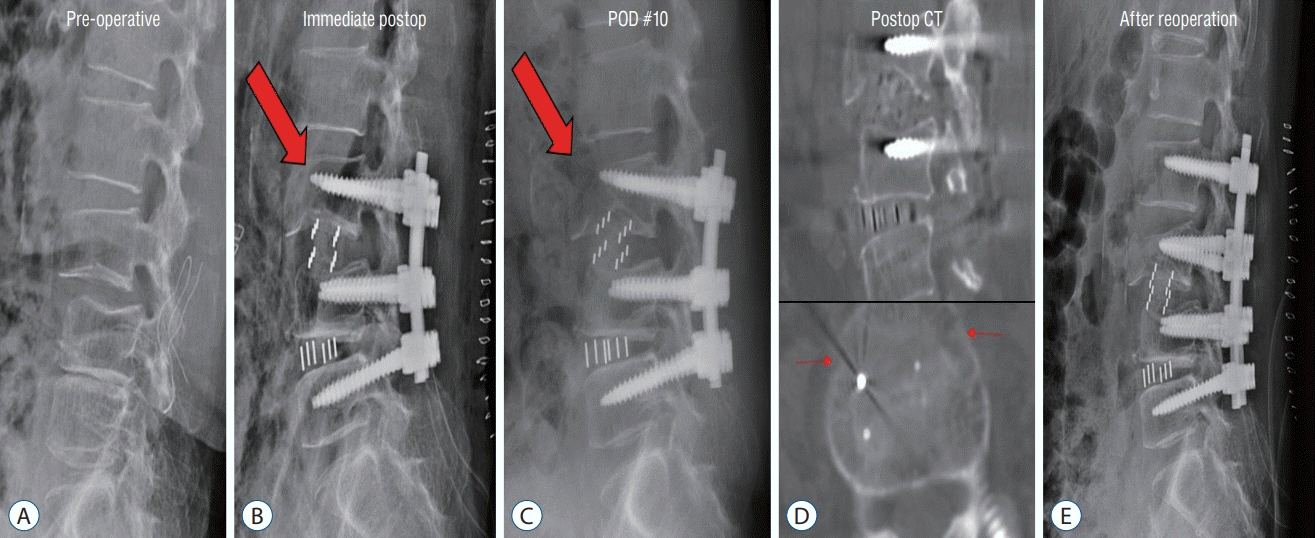 | Fig. 5.Case of vertebral body fracture (red arrow) requiring revision surgery. Preoperative (A), Immediate postoperative (B); L3 anterior vertebral body fracture. C : POD 10 day (POD #10); segmental kyphosis progressed. D : Postoperative CT; the coronal vertebral body fracture of L3. E : After reoperation; posterior fixation extending to L2. POD : postoperative day, CT : computed tomography. |
Injury to the ureter
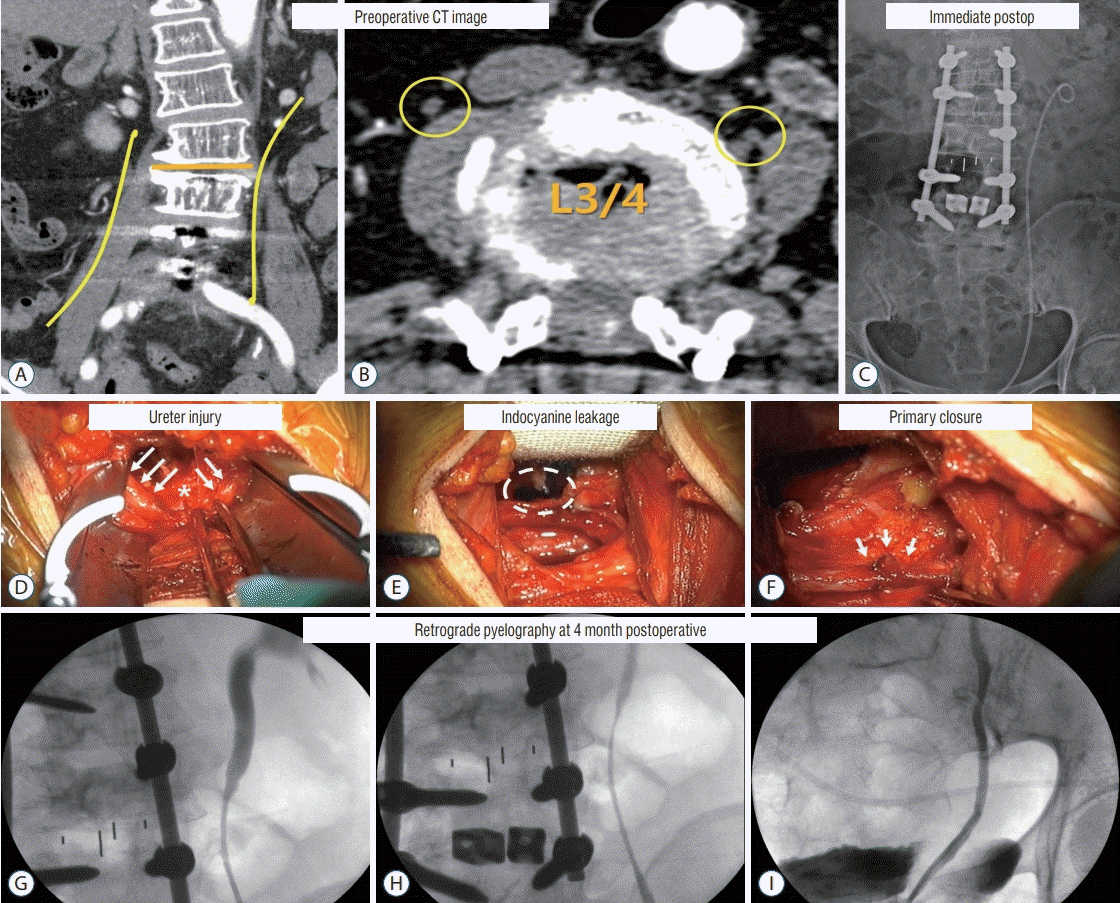 | Fig. 6.Case with a complication of injury to the ureter. Preoperative CT image. A : The course of the ureter is indicated by the yellow line. B : The ureter at the L3/4 area is marked with yellow circles. The right ureter is located above the psoas muscle, while the left ureter is located at the border of the vertebral body and the psoas muscle. Immediate postoperative image. C : L3–4 1 level OLIF with L1–L5 posterior fusion, left ureter with double J catheter inserted. Intraoperative images. D : Ureter (white arrows), injury site (asterisk). E : Indocyanine leakage was found at the site of injury of the ureter (dotted circle). F : A double J catheter was inserted, and primary closure was implemented (white arrows). Four months postoperative retrograde pyelography. G-I : Mild stenosis at the suture site; however, the contrast passage was good. CT : computed tomography, OLIF : oblique lateral interbody fusion. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download