1. Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, Yarascavitch B, et al. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg. 259:449–457. 2014.
2. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 286:992–999. 2018.

3. Beck J, Gralla J, Fung C, Ulrich CT, Schucht P, Fichtner J, et al. Spinal cerebrospinal fluid leak as the cause of chronic subdural hematomas in nongeriatric patients. J Neurosurg. 121:1380–1387. 2014.

4. Beck J, Häni L, Ulrich CT, Fung C, Jesse CM, Piechowiak E, et al. : Diagnostic challenges and therapeutic possibilities in spontaneous intracranial hypotension. Clin Transl Neurosci 2 : 2514183X18787371, 2018. Beck J, Häni L, Ulrich CT, Fung C, Jesse CM, Piechowiak E, et al. Diagnostic challenges and therapeutic possibilities in spontaneous intracranial hypotension. Clin Transl Neurosci. 2:2514183X18787371. 2018.

5. Berroir S, Loisel B, Ducros A, Boukobza M, Tzourio C, Valade D, et al. Early epidural blood patch in spontaneous intracranial hypotension. Neurology. 63:1950–1951. 2004.

6. Busl KM, Prabhakaran S. Predictors of mortality in nontraumatic subdural hematoma. J Neurosurg. 119:1296–1301. 2013.

7. Chen HH, Huang CI, Hseu SS, Lirng JF. Bilateral subdural hematomas caused by spontaneous intracranial hypotension. J Chin Med Assoc. 71:147–151. 2008.

8. Chen YC, Wang YF, Li JY, Chen SP, Lirng JF, Hseu SS, et al. Treatment and prognosis of subdural hematoma in patients with spontaneous intracranial hypotension. Cephalalgia. 36:225–231. 2016.

9. Chung SJ, Lee JH, Kim SJ, Kwun BD, Lee MC. Subdural hematoma in spontaneous CSF hypovolemia. Neurology. 67:1088–1089. 2006.

10. Dehaene S, Biesemans J, Van Boxem K, Vidts W, Sterken J, Van Zundert J. Post-dural puncture headache evolving to a subdural hematoma: a case report. Pain Pract. 21:83–87. 2021.

11. Dhillon AK, Rabinstein AA, Wijdicks EF. Coma from worsening spontaneous intracranial hypotension after subdural hematoma evacuation. Neurocrit Care. 12:390–394. 2010.

12. Ferrante E, Arpino I, Citterio A, Savino A. Coma resulting from spontaneous intracranial hypotension treated with the epidural blood patch in the trendelenburg position pre-medicated with acetazolamide. Clin Neurol Neurosurg. 111:699–702. 2009.

13. Ferrante E, Olgiati E, Sangalli V, Rubino F. Early pain relief from orthostatic headache and hearing changes in spontaneous intracranial hypotension after epidural blood patch. Acta Neurol Belg. 116:503–508. 2016.

14. Ferrante E, Trimboli M, Pontrelli G, Rubino F. Early coma awakening after epidural blood patch. J Clin Neurosci. 71:295–296. 2020.

15. Frontera JA, Egorova N, Moskowitz AJ. National trend in prevalence, cost, and discharge disposition after subdural hematoma from 1998-2007. Crit Care Med. 39:1619–1625. 2011.

16. Gordon N. Spontaneous intracranial hypotension. Dev Med Child Neurol. 51:932–935. 2009.

17. Hashizume K, Watanabe K, Kawaguchi M, Fujiwara A, Furuya H. Evaluation on a clinical course of subdural hematoma in patients undergoing epidural blood patch for spontaneous cerebrospinal fluid leak. Clin Neurol Neurosurg. 115:1403–1406. 2013.

18. Kim BR, Lee JW, Lee E, Kang Y, Ahn JM, Kang HS. Utility of heavily T2-weighted MR myelography as the first step in CSF leak detection and the planning of epidural blood patches. J Clin Neurosci. 77:110–115. 2020.

19. Kim J, Moon J, Kim T, Ahn S, Hwang G, Bang J, et al. Risk factor analysis for the recurrence of chronic subdural hematoma: a review of 368 consecutive surgical cases. Korean J Neurotrauma. 11:63–69. 2015.

20. Klein KM, Shiratori K, Knake S, Hamer HM, Fritsch B, Todorova-Rudolph A, et al. Status epilepticus and seizures induced by iopamidol myelography. Seizure. 13:196–199. 2004.

21. Louhab N, Adali N, Laghmari M, Hymer WE, Ben Ali SA, Kissani N. Misdiagnosed spontaneous intracranial hypotension complicated by subdural hematoma following lumbar puncture. Int J Gen Med. 7:71–73. 2014.

22. Mao YT, Dong Q, Fu JH. Delayed subdural hematoma and cerebral venous thrombosis in a patient with spontaneous intracranial hypotension. Neurol Sci. 32:981–983. 2011.

23. Perez-Vega C, Robles-Lomelin P, Robles-Lomelin I, Diaz-Alba A, Navarro VG. Acute subdural hematoma recurrence during drain removal associated with spontaneous intracranial hypotension - a non-reported complication. Surg Neurol Int. 11:316. 2020.

24. Sayer FT, Bodelsson M, Larsson EM, Romner B. Spontaneous intracranial hypotension resulting in coma: case report. Neurosurgery. 59:E204. discussion E204. 2006.

25. Schievink WI. Stroke and death due to spontaneous intracranial hypotension. Neurocrit Care. 18:248–251. 2013.

26. Schievink WI, Maya MM, Moser F, Tourje J, Torbati S. Frequency of spontaneous intracranial hypotension in the emergency department. J Headache Pain. 8:325–328. 2007.

27. Schievink WI, Maya MM, Moser FG, Tourje J. Spectrum of subdural fluid collections in spontaneous intracranial hypotension. J Neurosurg. 103:608–613. 2005.

28. Schievink WI, Maya MM, Pikul BK, Louy C. Spontaneous spinal cerebrospinal fluid leaks as the cause of subdural hematomas in elderly patients on anticoagulation. J Neurosurg. 112:295–299. 2010.

29. Souirti Z, Benzagmout M, Belahsen F, Chaoui ME. Spontaneous bilateral subacute subdural hematoma revealing intracranial hypotension. Neurosciences (Riyadh). 14:384–385. 2009.
30. Takahashi K, Mima T, Akiba Y. Chronic subdural hematoma associated with spontaneous intracranial hypotension: therapeutic strategies and outcomes of 55 cases. Neurol Med Chir (Tokyo). 56:69–76. 2016.

31. Tsai PH, Fuh JL, Lirng JF, Wang SJ. Heavily T2-weighted MR myelography in patients with spontaneous intracranial hypotension: a case-control study. Cephalalgia. 27:929–934. 2007.

32. Tsai PH, Fuh JL, Lirng JF, Wang SJ. Comparisons between heavily T2-weighted MR and CT myelography studies in two patients with spontaneous intracranial hypotension. Cephalalgia. 28:653–657. 2008.

33. Wang YF, Lirng JF, Fuh JL, Hseu SS, Wang SJ. Heavily T2-weighted MR myelography vs CT myelography in spontaneous intracranial hypotension. Neurology. 73:1892–1898. 2009.

34. Whiteley W, Al-Shahi R, Myles L, Lueck CJ. Spontaneous intracranial hypotension causing confusion and coma: a headache for the neurologist and the neurosurgeon. Br J Neurosurg. 17:456–458. 2003.

35. Yadav YR, Parihar V, Namdev H, Bajaj J. Chronic subdural hematoma. Asian J Neurosurg. 11:330–342. 2016.

36. Yoon SH, Chung YS, Yoon BW, Kim JE, Paek SH, Kim DG. Clinical experiences with spontaneous intracranial hypotension: a proposal of a diagnostic approach and treatment. Clin Neurol Neurosurg. 113:373–379. 2011.

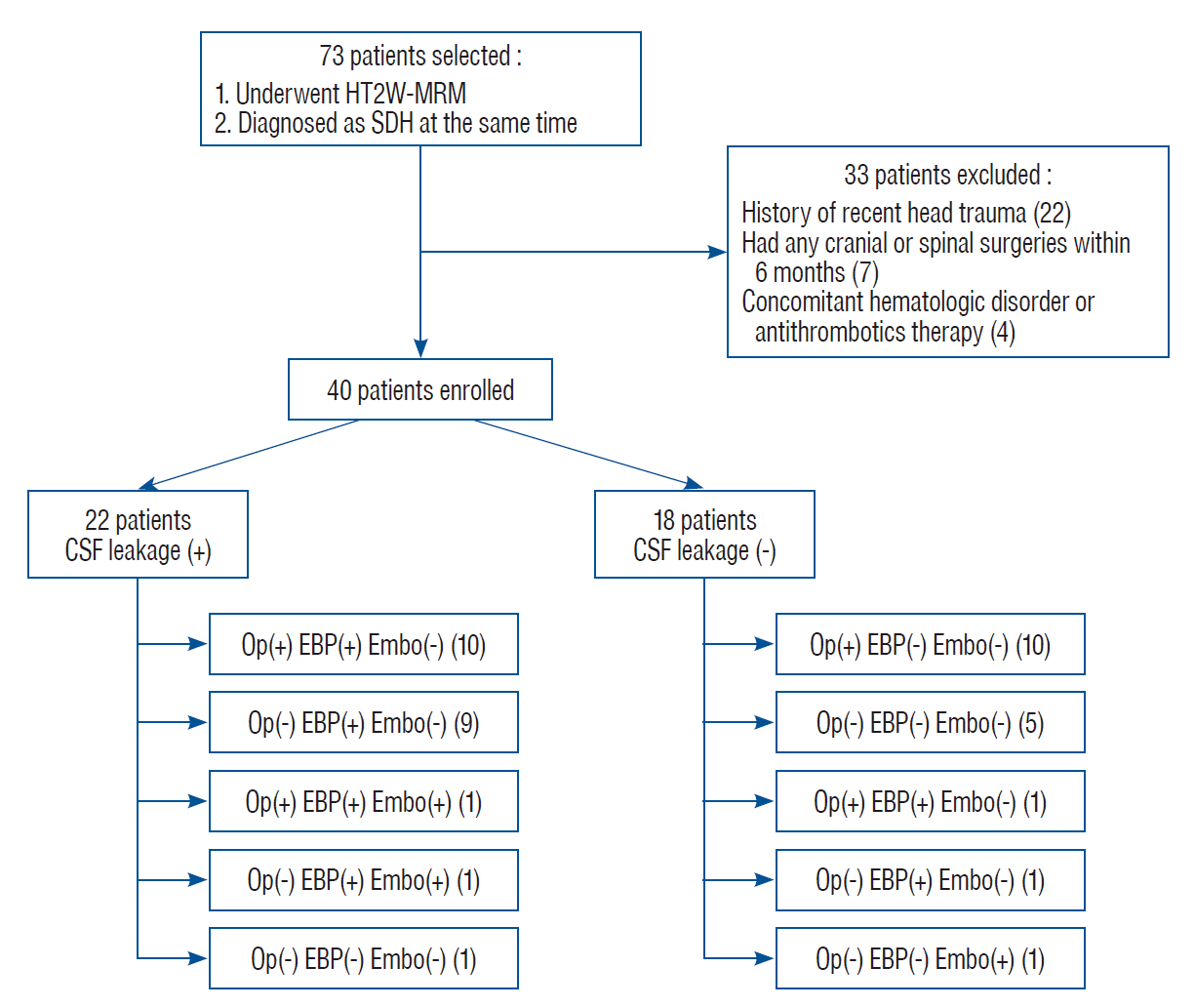
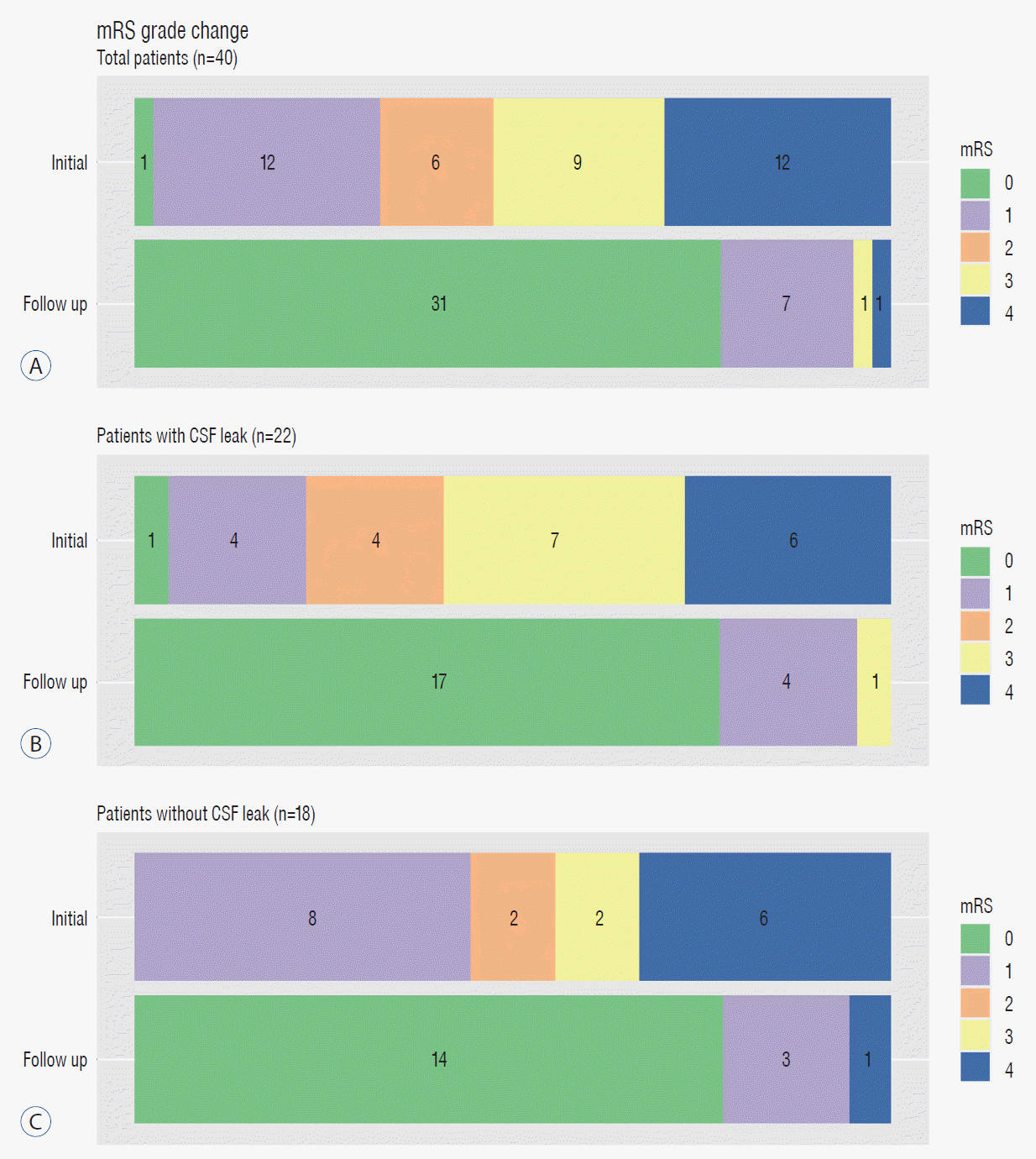




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download