INTRODUCTION
MATERIALS AND METHODS
Animal models and care
Experimental groups
Table 1.
Group 1 is the negative control group without mechanical thrombectomy (MT). MT was performed on experimental day 8 for the group 2, group 3, and group 4 rabbits. Group 2 is the positive control group without statin administration. We administered 20 mg atorvastatin daily to the group 3 (7 days before MT, day 1–7) and group 4 (7 days after MT, day 8–14) rabbits. On day 15, the rabbits of all groups were euthanized
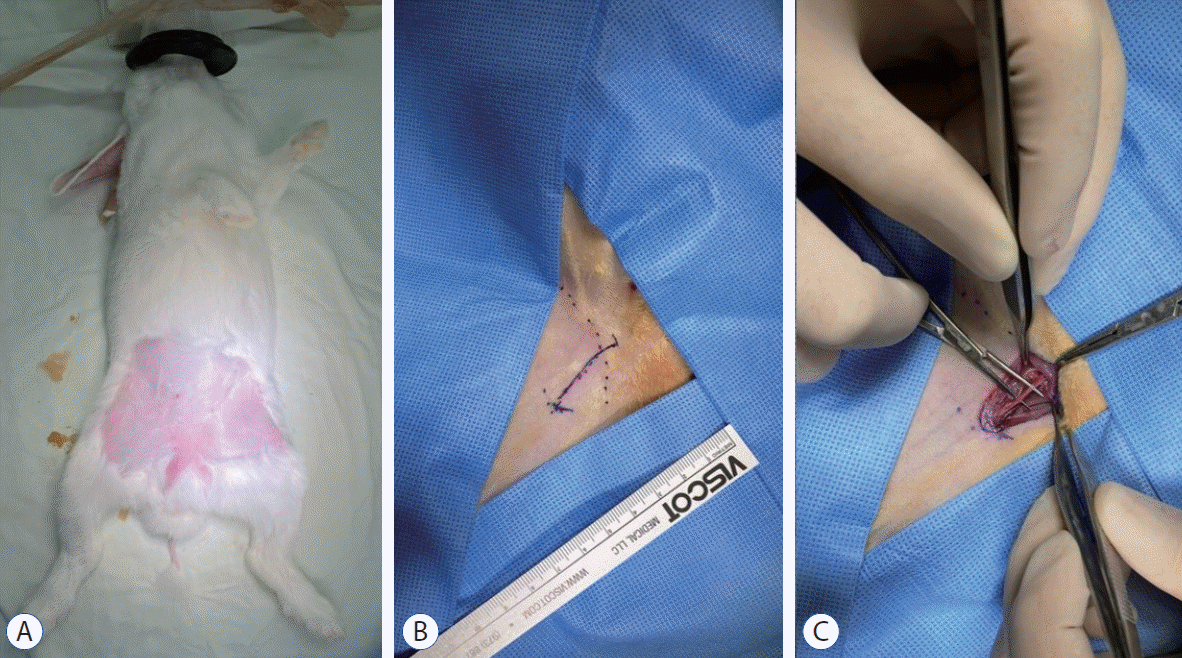
Procedure for endovascular MT
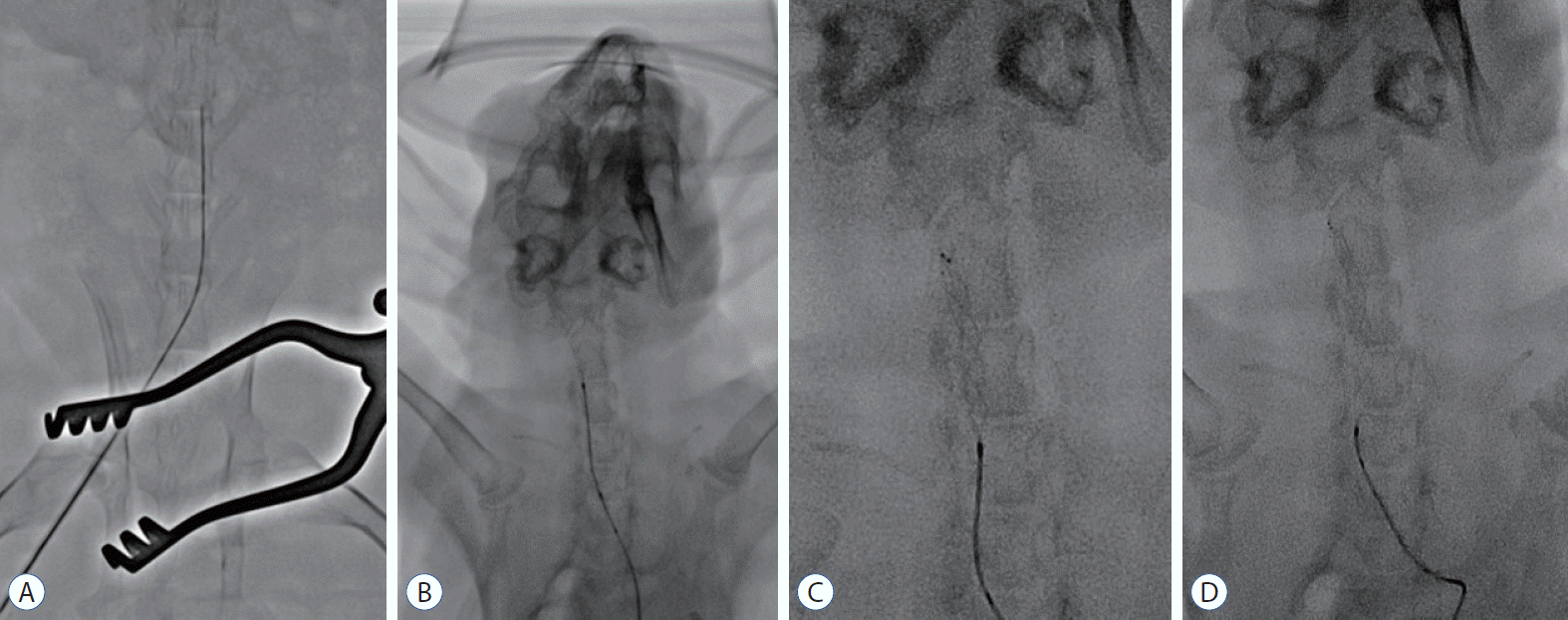 | Fig. 2.Procedure for endovascular MT using stent retrievers in the rabbit. The microcatheter was navigated via the right femoral artery to the distal carotid artery through a 4 F sheath (A). A stent retriever was introduced into the microcatheter and deployed in the carotid artery (b). For tight apposition of the stent to the vessel wall, we used the ‘push and fluff technique’ while deploying the stent (c). After 2–3 minutes, the deployed stent was pulled back to the proximal segment of the carotid artery (d). |
Histological and immunohistochemical analyses
Statistical analysis
RESULTS
Histomorphological analysis of the tunica intima
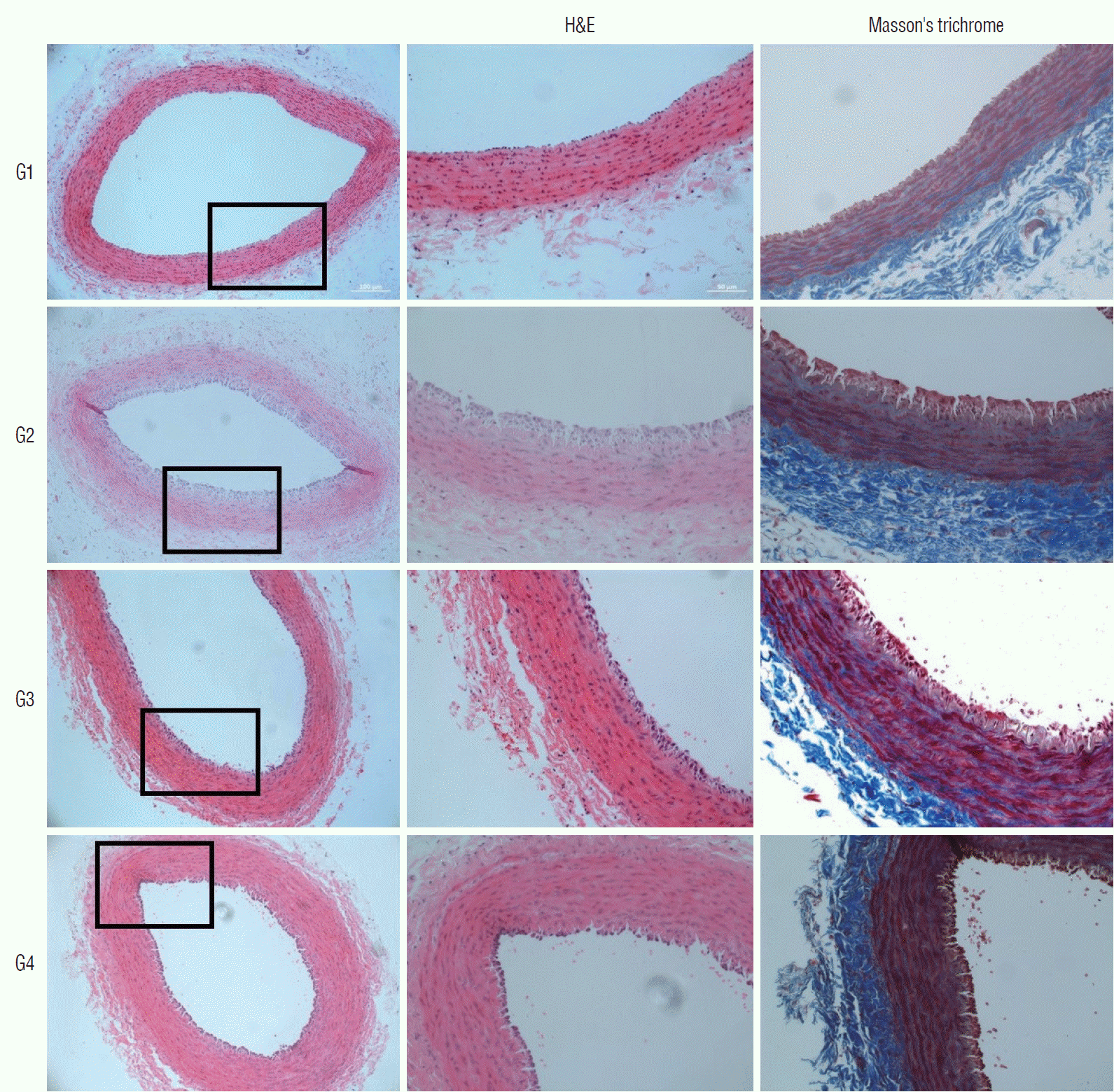 | Fig. 3.Microscopic view of the H&E and Masson’s trichrome stained tissues. The tunica intima of the negative control group (group 1) shows a single thin layer of endothelial cells. Marked thickening of tunica intima can be observed in the positive control group (group 2). In the statin-administered groups 3 and 4, a significant decrease in intimal thickening can be observed (magnification ×20 on 1st column, magnification ×200 on 2nd and 3rd columns). H&E : hematoxylin and eosin, G1 : group 1, G2 : group 2, G3 : group 3, G4 : group 4. |
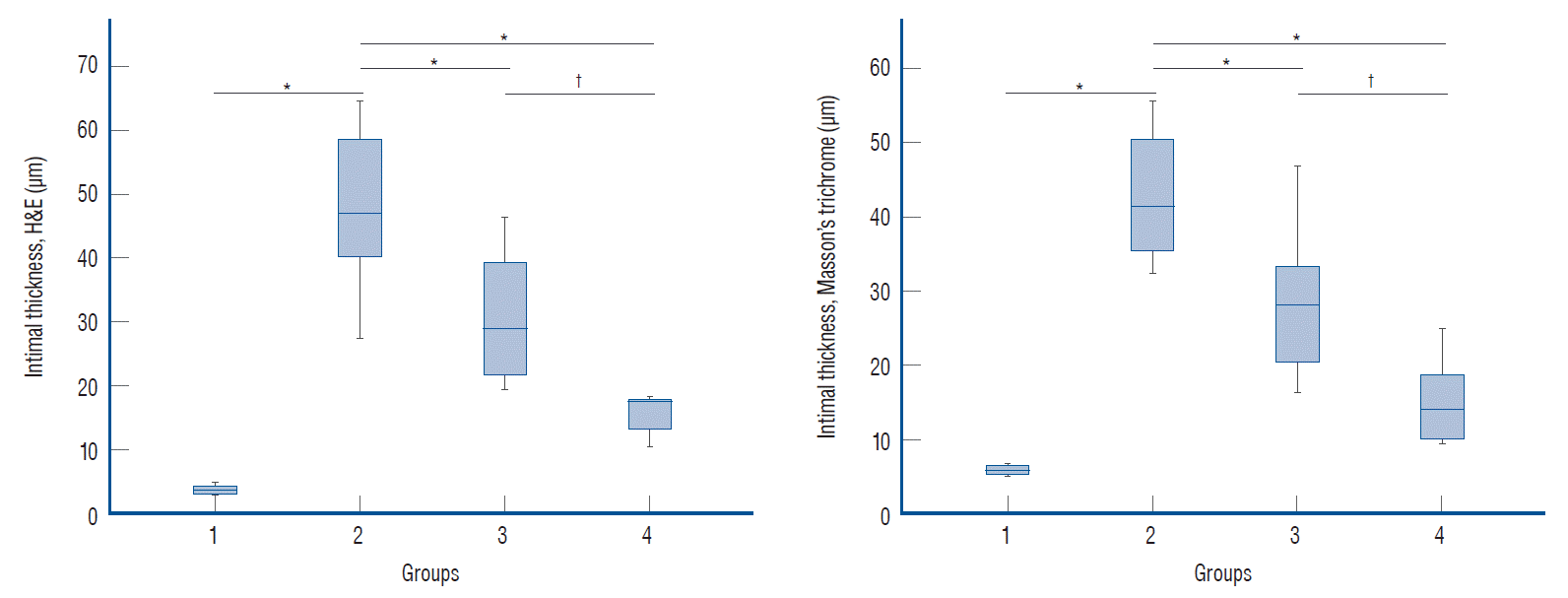 | Fig. 4.Histomorphological analysis of the intimal thickness in the hematoxylin and eosin (H&E) and Masson’s trichrome stained tissues. Marked intimal thickening was observed in the groups for which endovascular mechanical thrombectomy was performed. compared to the positive control group (group 2), the statin-administered groups (groups 3 and 4) showed decreased intimal thickness. The statistical analysis was performed using one-way analysis of variance (ANOVA) for three or more groups. Post-hoc was performed using duncan’s method. *p<0.05 vs. the positive control group. †p<0.05 between the statin-administered groups (before and after endovascular mechanical thrombectomy). |
Table 2.
| Group 1 (n=4) | Group 2 (n=8) | Group 3 (n=8) | Group 4 (n=8) | F* | Sig.(p)* | |
|---|---|---|---|---|---|---|
| Intimal thickness (µm) | ||||||
| H&E | 3.74±0.62a | 48.04±12.28d | 30.88±10.41c | 15.86±3.19b | 28.885 | 0.001 |
| Masson’s trichrome | 5.84±0.63a | 42.72±8.82d | 28.36±9.86c | 14.93±5.75b | 26.754 | 0.001 |
| Immunohistochemistry (area%) | ||||||
| TNF-α | 10.55±3.63a | 21.75±6.78c | 15.55±3.44a,b | 18.46±3.44b,c | 5.703 | 0.004 |
| CD11b | 11.15±4.95a | 19.64±5.19b | 14.84±3.63a,b | 14.35±5.27a,b | 3.303 | 0.037 |
| CD163 | 14.25±1.28 | 24.54±8.59 | 17.65±5.86 | 17.47±6.62 | 2.758 | 0.064 |
Immunohistochemical analysis of the statin effect
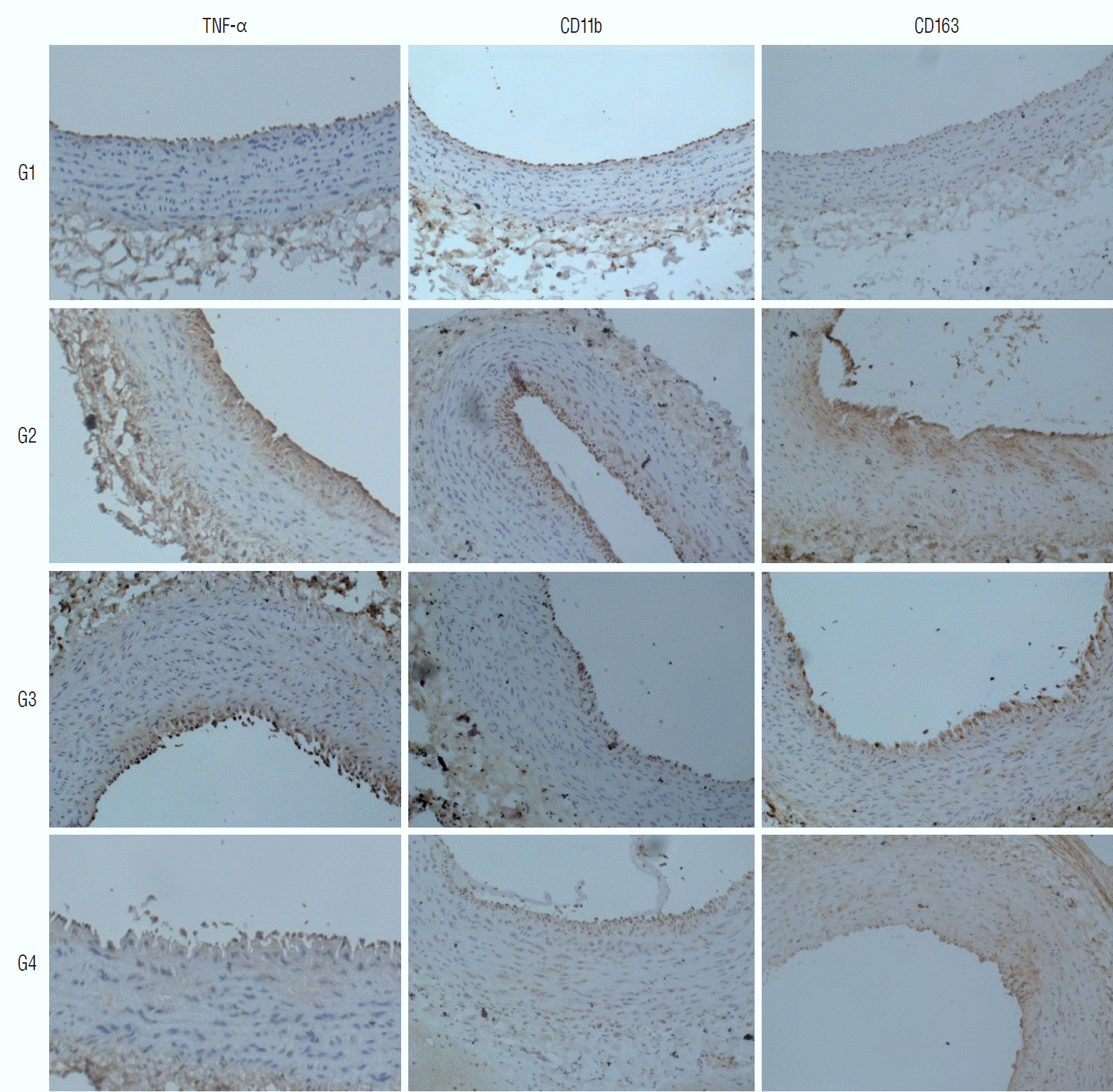 | Fig. 5.Microscopic view of the immunohistochemically stained tissues. The stained percentage areas were markedly increased in the positive control group (group 2), compared to in the negative control group (group 1). Statin administration reduced the observed percentage areas of all immunological antibodies (magnification ×200 for all samples). TNF-α : tumor necrosis factor-α, cd : cluster of differentiation, G1 : group 1, G2 : group 2, G3 : group 3, G4 : group 4. |
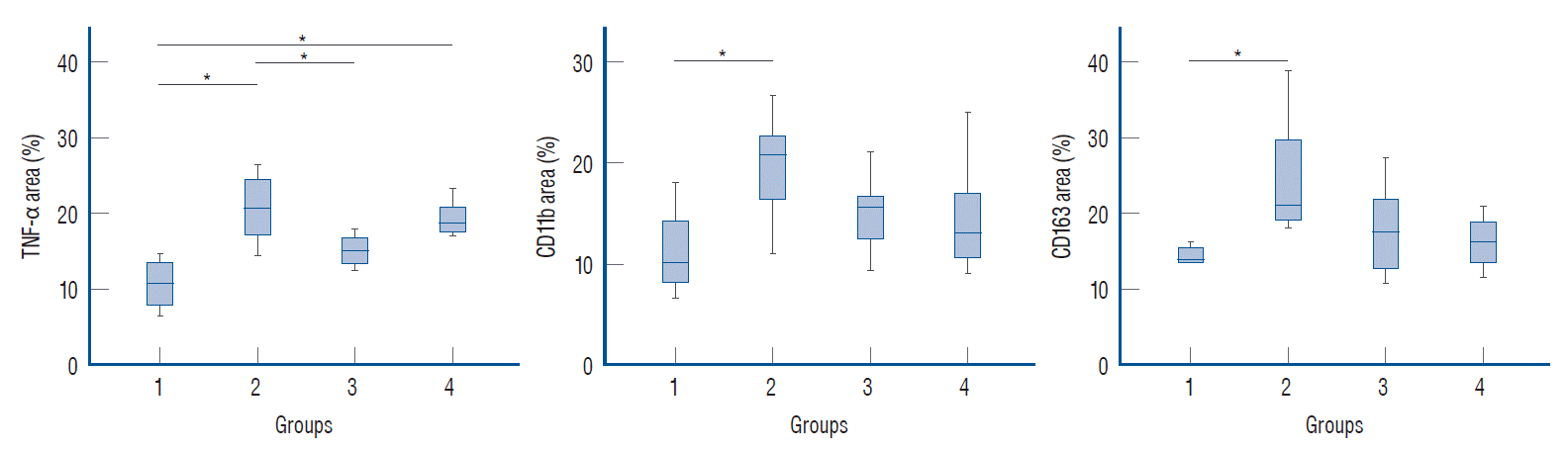 | Fig. 6.Immunohistochemical analysis of the inflammatory markers. The percentage area of all immunological antibodies significantly increased in positive control group (group 2), compared to in the negative control group (group 1). In group 3, the percentage area of TNF-α staining was significantly reduced (p<0.05), compared to the positive control group (group 2), but significant differences were not observed for cd11b and cd163 staining. In group 4, no significant differences were observed for TNF-α, cd11b, and cd163 staining (p≥0.05). The statistical analysis was performed using one-way analysis of variance (ANOVA) for three or more groups. *p<0.05 vs. positive control group. TNF-α : tumor necrosis factor-α, cd : cluster of differentiation. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download