INTRODUCTION
MATERIALS AND METHODS
RESULTS
Table 1.
| Variable | Value (n=23) |
|---|---|
| Sex, M : F | 15 : 8 |
| Age at admission (years) | 68.9 (39–94) |
| Initial symptom | |
| Pain (axial or extremity) | 12 (52.2) |
| Fever | 6 (26.1) |
| Neurologic deficit | 3 (13.0) |
| Miscellaneous | 2 (8.7) |
| Time from symptom onset to admission (days) | 10.3 (0–120) |
| Comorbidity and past medical history | |
| Hypertension | 12 (52.2) |
| Diabetes mellitus | 10 (43.5) |
| Stroke history | 6 (26.1) |
| Heart problem | 5 (21.7) |
| Chronic kidney disease | 3 (13.0) |
| Malignancy history | 3 (13.0) |
| Concomitant infection | 12 (52.2) |
| Invasive spinal intervention history* | |
| Injection or acupuncture | 6 (26.1) |
| Open spine surgery | 1 (4.3) |
| Vertebroplasty+injection | 1 (4.3) |
| Time from admission to diagnosis (days) | |
| Bacteremia by blood culture† | 1.7 (0–19) |
| Radiologic diagnosis by MRI | 8.4 (0–59) |
| Time from admission to operation (days) | 16.6 (0–66) |
| Microbiological data | |
| Positive peripheral blood culture | 23 (100.0) |
| Positive surgical specimen culture | 15 (65.2) |
| Radiologic data (from immediate preoperative MRI) | |
| Cervical spine abscess | 4 (17.4) |
| Thoracic spine abscess | 2 (8.7) |
| Thoraco-lumbar spine abscess | 2 (8.7) |
| Lumbar spine abscess | 11 (47.8) |
| Lumbo-sacral spine abscess | 4 (17.4) |
| Osteomyelitis | 18 (78.3) |
| Psoas or paraspinal abscess | 15 (65.2) |
| Size (vertebral bodies) | 2.9 (1–6) |
| Severity of thecal sac compression‡ | |
| Mild | 5 (21.7) |
| Moderate | 12 (52.2) |
| Severe | 6 (26.1) |
| Location | |
| Ventral | 14 (60.9) |
| Dorsal | 4 (17.4) |
| Both | 5 (21.7) |
| Recurrence | 1 (4.3) |
| Mortality | 3 (13.0) |
Table 2.
| No. | Sex/age* | Initial symptom |
Time to† (days) |
Comorbidity | Past medical history |
Neurologic status‡ (mRS) |
Mode of Op. |
Duration of antibiotics (days) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adm | Dx | Op | Adm | Preop | Final | Preop. | Postop. | ||||||
| 1 | M/76 | Fever | 2 | 1 | 32 | DM | Prostate Ca. | 1 | 1 | 0 | Lami | 32 | 25 |
| 2 | M/70 | Weakness | 7 | 0 | 5 | CABG, CVA | 2 | 2 | 0 | Lami | 0 | 76 | |
| 3 | F/58 | Back pain | 7 | 0 | 1 | 0 | 0 | 0 | Lami | 0 | 54 | ||
| 4 | F/64 | Back pain | 21 | 0 | 27 | HT | 0 | 0 | 0 | Lami | 27 | 44 | |
| 5 | F/75 | Back pain | 6 | 0 | 4 | DM, MI | CVA | 1 | 3 | 6 | Lami | 4 | 38 |
| 6 | M/53 | Fever | 2 | 25 | 30 | DM, dementia | CVA | 0 | 0 | 0 | Lami | 30 | 50 |
| 7 | F/76 | Back pain | 7 | 7 | 11 | HT | 3 | 3 | 3 | Lami | 0 | 64 | |
| 8 | M/68 | Back pain | 0 | 59 | 66 | DM, HT | 0 | 0 | 0 | Lami | 46 | 23 | |
| 9 | M/85 | Back pain | 5 | 0 | 8 | HT, hypothyroidism | 4 | 4 | 6 | Lami | 8 | 23 | |
| 10 | M/65 | Back pain | 120 | 1 | 2 | DM, HT | 2 | 2 | 0 | Retro | 0 | 58 | |
| 11 | F/79 | Fever | 2 | 12 | 23 | HT, Af, MI | CVA, Rectal Ca. | 4 | 4 | 0 | Lami | 21 | 72 |
| 12 | M/59 | Fever | 2 | 7 | 9 | HT | 3 | 3 | 0 | Lami | 9 | 95 | |
| 13 | M/52 | Back pain | 2 | 20 | 27 | 0 | 0 | 0 | Lami | 27 | 23 | ||
| 14 | F/71 | Back pain | 6 | 28 | 43 | DM, HT, CKD | AVR, CVA | 0 | 0 | 0 | Lami | 47 | 23 |
| 15 | F/90 | Dyspnea | 1 | 3 | 14 | HT | 0 | 0 | 0 | Lami | 7 | 36 | |
| 16 | M/81 | Weakness | 0 | 0 | 0 | DM | 4 | 5 | 3 | Lami | 0 | 49 | |
| 17 | F/55 | Back pain | 7 | 1 | 2 | 2 | 2 | 0 | Lami | 1 | 67 | ||
| 18 | M/39 | Melena | 3 | 2 | 7 | Mental retardation | 0 | 0 | 0 | Lami | 7 | 68 | |
| 19 | M/72 | Weakness | 7 | 0 | 2 | CVA | 5 | 5 | 5 | Corp | 2 | 48 | |
| 20 | M/67 | Back pain | 4 | 0 | 1 | DM, HT, CKD | Kidney TPL | 2 | 2 | 0 | Lami | 0 | 45 |
| 21 | M/94 | Fever | 2 | 0 | 26 | DM, HT | Gastric Ca. | 0 | 0 | 0 | Lami | 26 | 64 |
| 22 | M/83 | Fever | 0 | 27 | 29 | DM, HT, ESRD, Af | 2 | 5 | 6 | Lami | 29 | 68 | |
| 23 | M/53 | Neck pain | 24 | 0 | 12 | 2 | 3 | 0 | Corp | 9 | 56 | ||
† Adm: time from initial symptom onset to admission, Dx : time from admission to radiologic diagnosis of epidural abscess using MRI, Op : time from admission to surgery for abscess removal.
‡ Adm : at admission, Preop : immediate preoperative, Final : at last follow-up. mRS : modified Rankin scale, Op. : operation, Preop. : duration of preoperative antibiotic treatment, Postop. : duration of postoperative antibiotic treatment, M : male, DM : diabetes mellitus, Ca. : cancer, Lami : laminectomy and abscess removal, CABG : coronary artery bypass graft, CVA : cerebrovascular accident, F : female, HT : hypertension, MI : myocardial infarction, Retro : retroperitoneal approach and abscess removal, Af : atrial fibrillation, CKD : chronic kidney disease, AVR : atrial valve replacement, Corp : corpectomy and abscess removal, TPL : transplantation, ESRD : end-stage renal disease
Table 3.
| No. | Previous intervention* | Concomitant infection |
Identified organism |
|
|---|---|---|---|---|
| Peripheral blood | Surgical specimen | |||
| 1 | S. agalactiae | No growth | ||
| 2 | lumbar fusion | MRSE | MRSE | |
| 3 | SAG+S. capitis | SAG | ||
| 4 | Injection | SAG | No growth | |
| 5 | UTI† | MSSA | MSSA | |
| 6 | Scrotal abscess†/UTI† | MRSA | MRSA | |
| 7 | MSSE+S. caprae | No growth | ||
| 8 | Scapular abscess | MRSA | No growth | |
| 9 | MSSA | MSSA+S. warneri | ||
| 10 | VP/injection | E. faecalis | E. faecalis | |
| 11 | Acupuncture | Urosepsis† | E. coli | E. coli |
| 12 | Injection | E. coli | E. coli | |
| 13 | Pl. eff.†/septic arthritis | MSSA | No growth | |
| 14 | Inf. endocarditis | MRSE | No growth | |
| 15 | Injection | Inf. endocarditis | E. faecalis | No growth |
| 16 | Injection | MSSA | MSSA | |
| 17 | UTI† | MSSA | MSSA | |
| 18 | MSSA | MSSA | ||
| 19 | UTI† | MSSA | MSSA | |
| 20 | S. agalactiae | S. agalactiae | ||
| 21 | Injection | UTI† | MRSA+MSSA | No growth |
| 22 | AV infection†/UTI† | MRSA | MRSA | |
| 23 | UTI† | MSSA | MSSA | |
S. agalactiae : Streptococcus agalactiae-(group B), MRSE : methicillin-resistant Staphylococcus epidermidis, SAG : Streptococcus anginosus group, S. capitis : Staphylococcus capitis, UTI : urinary tract infection, MSSA : methicillin-sensitive Staphylococcus aureus, MRSA : methicillinresistant Staphylococcus aureus, MSSE : methicillin-sensitive Staphylococcus epidermidis, S. caprae : Staphylococcus caprae, S. warneri : Staphylococcus warneri, VP : vertebroplasty, E. faecalis : Enterococcus faecalis, E. coli : Escherichia coli, Pl. eff. : pleural effusion, Inf. : infective, AV : arteriovenous graft
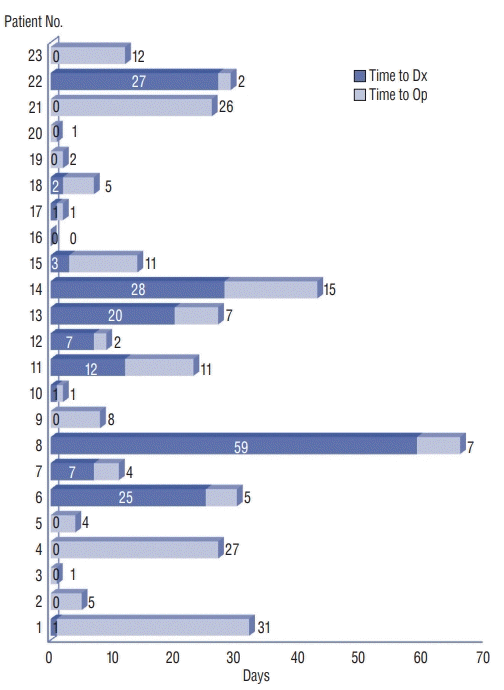 | Fig. 1.Time interval from admission to radiological diagnosis and operation (days). Each bar graph represents each patient’s time interval from admission to radiological diagnosis and operation. Time to Dx : time interval from admission to radiological diagnosis using magnetic resonance imaging, Time to Op : time interval from radiological diagnosis to operation. |
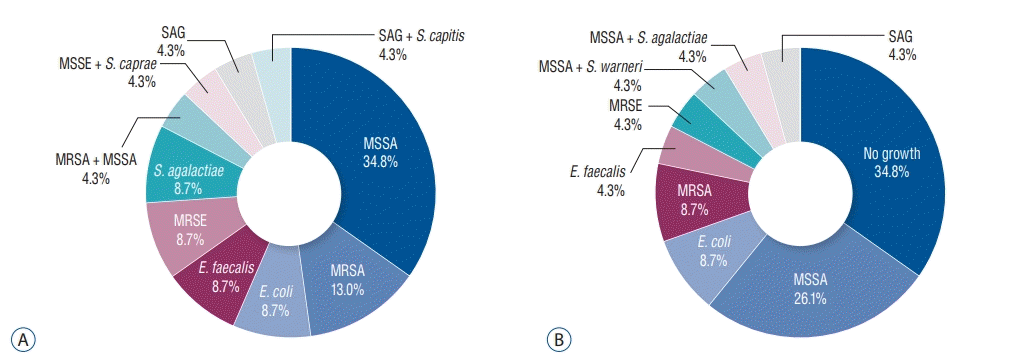 | Fig. 2.Identified organisms on microbiological study in peripheral blood culture (A), and in surgical specimen culture (B). MRSA : methicillin-resistant Staphylococcus aureus, MSSA : methicillin-sensitive Staphylococcus aureus, MSSE : methicillin-sensitive Staphylococcus epidermidis, S. caprae : Staphylococcus caprae, SAG : Streptococcus anginosus group, S. capitis : Staphylococcus capitis, S. agalactiae : Streptococcus agalactiae-(group B), MRSE : methicillin-resistant Staphylococcus epidermidis, E. faecalis : Enterococcus faecalis, E. coli : Escherichia coli, S. warneri : Staphylococcus warner. |
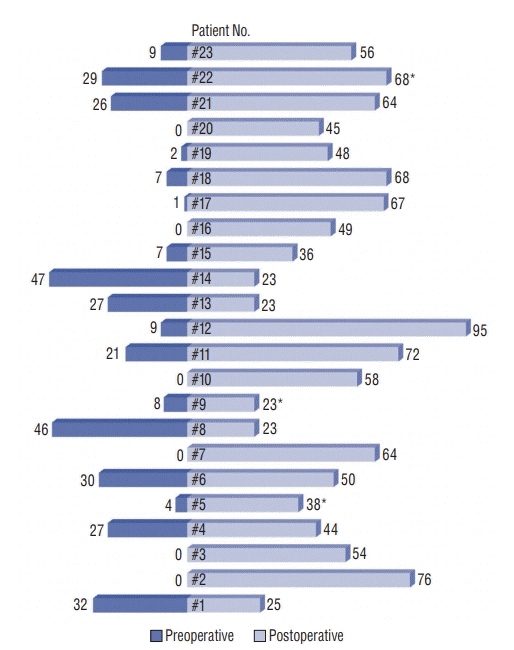 | Fig. 3.Duration of pre- and postoperative antibiotic treatment (days). Each bar graph represents each patient’s duration of pre- and postoperative antibiotic treatment. *Three patients (#5, #9, and #22) died and consequently their postoperative antibiotic treatments were not fully completed. Preoperative : duration of preoperative antibiotic treatment, Postoperative : duration of postoperative antibiotic treatment. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download