1. Aaron RK, Ciombor DM, Simon BJ. Treatment of nonunions with electric and electromagnetic fields. Clin Orthop Relat Res. 419:21–29. 2004.

3. Akhter S, Qureshi AR, Aleem I, El-Khechen HA, Khan S, Sikder O, et al. Efficacy of electrical stimulation for spinal fusion: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 10:4568. 2020.

4. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 328:1490. 2004.

5. Baumgart M, Wiśniewski M, Grzonkowska M, Małkowski B, Badura M, Dąbrowska M, et al. Digital image analysis of ossification centers in the axial dens and body in the human fetus. Surg Radiol Anat. 38:1195–1203. 2016.

7. Bose B. Outcomes after posterolateral lumbar fusion with instrumentation in patients treated with adjunctive pulsed electromagnetic field stimulation. Adv Ther. 18:12–20. 2001.

8. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3 Suppl. 3(Suppl 3):S131–S139. 2008.

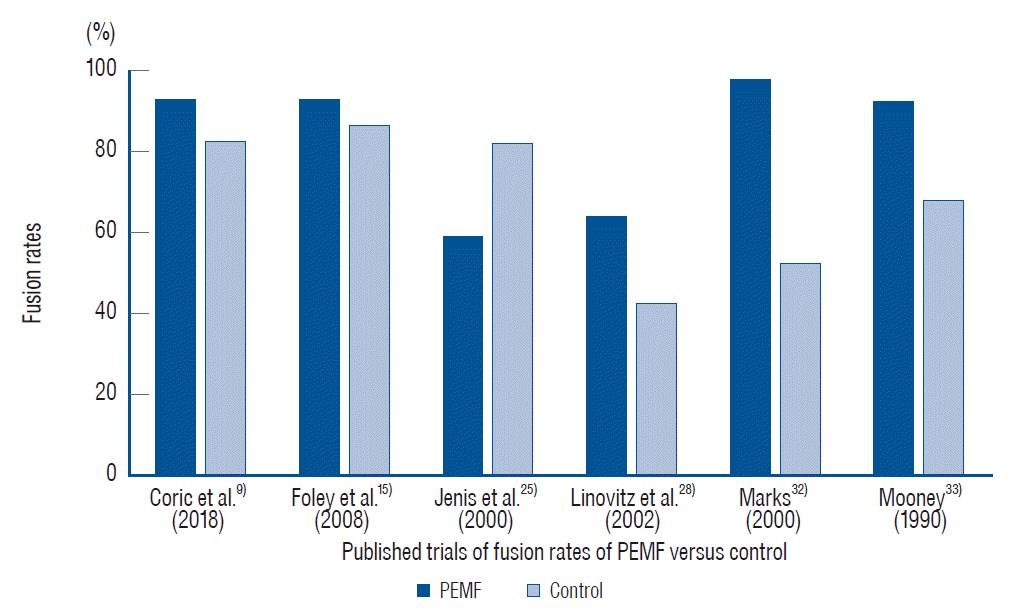
9. Coric D, Bullard DE, Patel VV, Ryaby JT, Atkinson BL, He D, et al. Pulsed electromagnetic field stimulation may improve fusion rates in cervical arthrodesis in high-risk populations. Bone Joint Res. 7:124–130. 2018.

10. Cottrill E, Pennington Z, Ahmed AK, Lubelski D, Goodwin ML, Perdomo-Pantoja A, et al. The effect of electrical stimulation therapies on spinal fusion: a cross-disciplinary systematic review and meta-analysis of the preclinical and clinical data. J Neurosurg Spine. 32:106–126. 2020.

12. Di Silvestre M, Savini R. Pulsing electromagnetic fields (PEMFs) in spinal fusion: preliminary clinical results. Chir Organi Mov. 77:289–294. 1992.
13. Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 9:66. 2011.

14. Doskocil M, Valouch P, Pazderka V. On vertebral body growth. Funct Dev Morphol. 3:149–155. 1993.
15. Foley KT, Mroz TE, Arnold PM, Chandler HC Jr, Dixon RA, Girasole GJ, et al. Randomized, prospective, and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J. 8:436–442. 2008.

16. Frykman GK, Taleisnik J, Peters G, Kaufman R, Helal B, Wood VE, et al. Treatment of nonunited scaphoid fractures by pulsed electromagnetic field and cast. J Hand Surg Am. 11:344–349. 1986.

17. Garland DE, Moses B, Salyer W. Long-term follow-up of fracture nonunions treated with PEMFs. Contemp Orthop. 22:295–302. 1991.
18. Gilbert SF. Developmental Biology. ed 6. Sunderland: Sinauer Associates;2000.
19. Goodwin CB, Brighton CT, Guyer RD, Johnson JR, Light KI, Yuan HA. A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine (Phila Pa 1976). 24:1349–1356. discussion 1357. 1999.

20. Griffin M, Bayat A. Electrical stimulation in bone healing: critical analysis by evaluating levels of evidence. Eplasty. 11:e34. 2011.
21. Gupta A, Kukkar N, Sharif K, Main BJ, Albers CE, El-Amin Iii SF. Bone graft substitutes for spine fusion: a brief review. World J Orthop. 6:449–456. 2015.

22. Hannemann PF, Essers BA, Schots JP, Dullaert K, Poeze M, Brink PR. Functional outcome and cost-effectiveness of pulsed electromagnetic fields in the treatment of acute scaphoid fractures: a cost-utility analysis. BMC Musculoskelet Disord. 16:84. 2015.

23. Huang AJ, Gemperli MP, Bergthold L, Singer SS, Garber A. Health plans’ coverage determinations for technology-based interventions: the case of electrical bone growth stimulation. Am J Manag Care. 10:957–962. 2004.
24. Huegel J, Choi DS, Nuss CA, Minnig MCC, Tucker JJ, Kuntz AF, et al. Effects of pulsed electromagnetic field therapy at different frequencies and durations on rotator cuff tendon-to-bone healing in a rat model. J Shoulder Elbow Surg. 27:553–560. 2018.

25. Jenis LG, An HS, Stein R, Young B. Prospective comparison of the effect of direct current electrical stimulation and pulsed electromagnetic fields on instrumented posterolateral lumbar arthrodesis. J Spinal Disord. 13:290–296. 2000.

26. Kahanovitz N. Electrical stimulation of spinal fusion: a scientific and clinical update. Spine J. 2:145–150. 2002.
27. Khalifeh JM, Zohny Z, MacEwan M, Stephen M, Johnston W, Gamble P, et al. Electrical stimulation and bone healing: a review of current technology and clinical applications. IEEE Rev Biomed Eng. 11:217–232. 2018.

28. Linovitz RJ, Pathria M, Bernhardt M, Green D, Law MD, McGuire RA, et al. Combined magnetic fields accelerate and increase spine fusion: a double-blind, randomized, placebo controlled study. Spine (Phila Pa 1976). 27:1383–1389. discussion 1389. 2002.
29. Makino T, Tsukazaki H, Ukon Y, Tateiwa D, Yoshikawa H, Kaito T. The biological enhancement of spinal fusion for spinal degenerative disease. Int J Mol Sci. 19:2430. 2018.

30. Markov MS. Expanding use of pulsed electromagnetic field therapies. Electromagn Biol Med. 26:257–274. 2007.

31. Markov MS. Pulsed electromagnetic field therapy history, state of the art and future. Environmentalist. 27:465–475. 2007.

32. Marks RA. Spine fusion for discogenic low back pain: outcomes in patients treated with or without pulsed electromagnetic field stimulation. Adv Ther. 17:57–67. 2000.

33. Mooney V. A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine (Phila Pa 1976). 15:708–712. 1990.

34. Morone MA, Feuer H. The use of electrical stimulation to enhance spinal fusion. Neurosurg Focus. 13:e5. 2002.

35. Oishi M, Onesti ST. Electrical bone graft stimulation for spinal fusion: a review. Neurosurgery. 47:1041–1055. discussion 1055-1056. 2000.

38. Park SB, Chung CK. Strategies of spinal fusion on osteoporotic spine. J Korean Neurosurg Soc. 49:317–322. 2011.

39. Rikk J, Finn KJ, Liziczai I, Radák Z, Bori Z, Ihász F. Influence of pulsing electromagnetic field therapy on resting blood pressure in aging adults. Electromagn Biol Med. 32:165–172. 2013.

40. Shupak NM, Prato FS, Thomas AW. Therapeutic uses of pulsed magnetic-field exposure: a review. URSI Radio Science Bulletin. 2003:9–32. 2003.
41. Simmons JW Jr, Mooney V, Thacker I. Pseudarthrosis after lumbar spine fusion: nonoperative salvage with pulsed electromagnetic fields. Am J Orthop (Belle Mead NJ). 33:27–30. 2004.
42. Strauch B, Herman C, Dabb R, Ignarro LJ, Pilla AA. Evidence-based use of pulsed electromagnetic field therapy in clinical plastic surgery. Aesthet Surg J. 29:135–143. 2009.

43. Tian NF, Wu YS, Zhang XL, Mao FM, Xu HZ, Chi YL. Efficacy of electrical stimulation for spinal fusion: a meta-analysis of fusion rate. Spine J. 13:1238–1243. 2013.

44. Victoria G, Petrisor B, Drew B, Dick D. Bone stimulation for fracture healing: what’s all the fuss? Indian J Orthop. 43:117–120. 2009.

45. Wade B. A review of pulsed electromagnetic field (PEMF) mechanisms at a cellular level: a rationale for clinical use. Am J Health Res. 1:51–55. 2013.

46. Waldorff EI, Zhang N, Ryaby JT. Pulsed electromagnetic field applications: a corporate perspective. J Orthop Translat. 9:60–68. 2017.

47. Wang C, Liu Y, Wang Y, Wei Z, Suo D, Ning G, et al. Low-frequency pulsed electromagnetic field promotes functional recovery, reduces inflammation and oxidative stress, and enhances HSP70 expression following spinal cord injury. Mol Med Rep. 19:1687–1693. 2019.

48. Wu N, Lee YC, Segina D, Murray H, Wilcox T, Boulanger L. Economic burden of illness among US patients experiencing fracture nonunion. Orthop Res Rev. 5:21–33. 2013.

49. Zborowski M, Androjna C, Waldorff EI, Midura RJ. Comparison of therapeutic magnetic stimulation with electric stimulation of spinal column vertebrae. IEEE Trans Magn. 51:1–9. 2015.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download