1. Abumi K. Cervical spondylotic myelopathy: posterior decompression and pedicle screw fixation. Eur Spine J 24 Suppl. 2:186–196. 2015.

2. Abumi K, Ito M, Sudo H. Reconstruction of the subaxial cervical spine using pedicle screw instrumentation. Spine (Phila Pa 1976). 37:E349–E356. 2012.

3. Ames CP, Smith JS, Scheer JK, Shaffrey CI, Lafage V, Deviren V, et al. A standardized nomenclature for cervical spine soft-tissue release and osteotomy for deformity correction: clinical article. J Neurosurg Spine. 19:269–278. 2013.

4. Bartolomei J, Sonntag V. Anterior approach including cervical corpectomy. In : Winn HR, Youmans JR, editors. Youmans Neurological Surgery. ed 5. Philadelphia: Saunders;2004. p. 4431–4445.
5. Chon H, Park JH. Cervical vertebral body fracture with ankylosing spondylitis treated with cervical pedicle screw: a fracture body overlapping reduction technique. J Clin Neurosci. 41:150–153. 2017.

6. Cunningham BW, Sefter JC, Shono Y, McAfee PC. Static and cyclical biomechanical analysis of pedicle screw spinal constructs. Spine (Phila Pa 1976). 18:1677–1688. 1993.

7. Dodwad SJ, Dodwad SN, Prasarn ML, Savage JW, Patel AA, Hsu WK. Posterior cervical foraminotomy: indications, technique, and outcomes. Clin Spine Surg. 29:177–185. 2016.
8. Fessler RG, Khoo LT. Minimally invasive cervical microendoscopic foraminotomy: an initial clinical experience. Neurosurgery. 51(5 Suppl):S37–S45. 2002.

9. Gu BS, Choi SJ, Yoo B, Han KH, Park JK, Lee YS, et al. An incidental finding of a radiopaque pill following cervical spinal surgery in a Parkinson’s disease patient. Korean J Spine. 12:153–155. 2015.

10. Gu BS, Park JH, Seong HY, Jung SK, Roh SW. Feasibility of posterior cervical foraminotomy in cervical foraminal stenosis: prediction of surgical outcomes by the foraminal shape on preoperative computed tomography. Spine (Phila Pa 1976). 42:E267–E271. 2017.
11. Gutman G, Rosenzweig DH, Golan JD. Surgical treatment of cervical radiculopathy: meta-analysis of randomized controlled trials. Spine (Phila Pa 1976). 43:E365–E372. 2018.
12. Hasegawa K, Hirano T, Shimoda H, Homma T, Morita O. Indications for cervical pedicle screw instrumentation in nontraumatic lesions. Spine (Phila Pa 1976). 33:2284–2289. 2008.

13. Heo Y, Lee SB, Lee BJ, Jeong SK, Rhim SC, Roh SW, et al. The learning curve of subaxial cervical pedicle screw placement: how can we avoid neurovascular complications in the initial period? Oper Neurosurg (Hagerstown). 17:603–607. 2019.

14. Johnston TL, Karaikovic EE, Lautenschlager EP, Marcu D. Cervical pedicle screws vs. lateral mass screws: uniplanar fatigue analysis and residual pullout strengths. Spine J. 6:667–672. 2006.

15. Kang MS, Choi KC, Lee CD, Shin YH, Hur SM, Lee SH. Effective cervical decompression by the posterior cervical foraminotomy without discectomy. J Spinal Disord Tech. 27:271–276. 2014.

16. Kerry G, Hammer A, Ruedinger C, Ranaie G, Steiner HH. Microsurgical posterior cervical foraminotomy: a study of 181 cases. Br J Neurosurg. 31:39–44. 2017.

17. Kim HB, Lee MK, Lee YS, Sohn JY, Jung SK, Park JH. An assessment of the medial angle of inserted subaxial cervical pedicle screw during surgery: practical use of preoperative CT scanning and intraoperative X-rays. Neurol Med Chir (Tokyo). 57:159–165. 2017.

18. Lee DH, Cho JH, Baik JM, Joo YS, Park S, Min WK, et al. Does additional uncinate resection increase pseudarthrosis following anterior cervical discectomy and fusion? Spine (Phila Pa 1976). 43:97–104. 2018.

19. Lee JK, Jung SK, Lee YS, Jeon SR, Roh SW, Rhim SC, et al. Analysis of the fusion and graft resorption rates, as measured by computed tomography, 1 year after posterior cervical fusion using a cervical pedicle screw. World Neurosurg. 99:171–178. 2017.

20. Lee S, Jung SK, Kim HB, Roh SW, Jeon SR, Park JH. Postoperative non-pathological fever following posterior cervical fusion surgery : is laminoplasty a better preventive method than laminectomy? J Korean Neurosurg Soc. 63:487–494. 2020.

21. Lee S, Seo J, Lee MK, Jeon SR, Roh SW, Rhim SC, et al. Widening of the safe trajectory range during subaxial cervical pedicle screw placement: advantages of a curved pedicle probe and laterally located starting point without creating a funnel-shaped hole. J Neurosurg Spine. 27:150–157. 2017.

22. Lee SH, Lee JS, Sung SK, Son DW, Lee SW, Song GS. The effect of uncinate process resection on subsidence following anterior cervical discectomy and fusion. J Korean Neurosurg Soc. 60:550–559. 2017.

23. Nakagawa H, Saito K, Mitsugi T, Yagi K, Kanno A. Microdiscectomy and foraminotomy in cervical spondylotic myelopathy and radiculopathy: anterior versus posterior, microendoscopic surgery versus mini-open microsurgery. World Neurosurg. 81:292–293. 2014.

24. Pakzaban P. Ultrasonic total uncinectomy: a novel technique for complete anterior decompression of cervical nerve roots. Neurosurgery 10 Suppl. 4:535–541. discussion 541. 2014.

25. Park JH, Jeon SR, Roh SW, Kim JH, Rhim SC. The safety and accuracy of freehand pedicle screw placement in the subaxial cervical spine: a series of 45 consecutive patients. Spine (Phila Pa 1976). 39:280–285. 2014.

26. Park JH, Roh SW, Rhim SC. A single-stage posterior approach with open reduction and pedicle screw fixation in subaxial cervical facet dislocations. J Neurosurg Spine. 23:35–41. 2015.

27. Park JH, Roh SW, Rhim SC. Response. J Neurosurg Spine. 24:673. 2016.
28. Polly DW Jr, Klemme WR, Cunningham BW, Burnette JB, Haggerty CJ, Oda I. The biomechanical significance of anterior column support in a simulated single-level spinal fusion. J Spinal Disord. 13:58–62. 2000.

29. Rho YJ, Choe WJ. Double crush syndrome caused by cervical spondylosis and vertebral artery loop. Eur Spine J. 28:292–296. 2019.

30. Roh SW, Kim DH, Cardoso AC, Fessler RG. Endoscopic foraminotomy using MED system in cadaveric specimens. Spine (Phila Pa 1976). 25:260–264. 2000.

31. Tan LA, Riew KD, Traynelis VC. Cervical spine deformity-part 3: posterior techniques, clinical outcome, and complications. Neurosurgery. 81:893–898. 2017.

32. Zeidman SM, Ducker TB. Posterior cervical laminoforaminotomy for radiculopathy: review of 172 cases. Neurosurgery. 33:356–362. 1993.

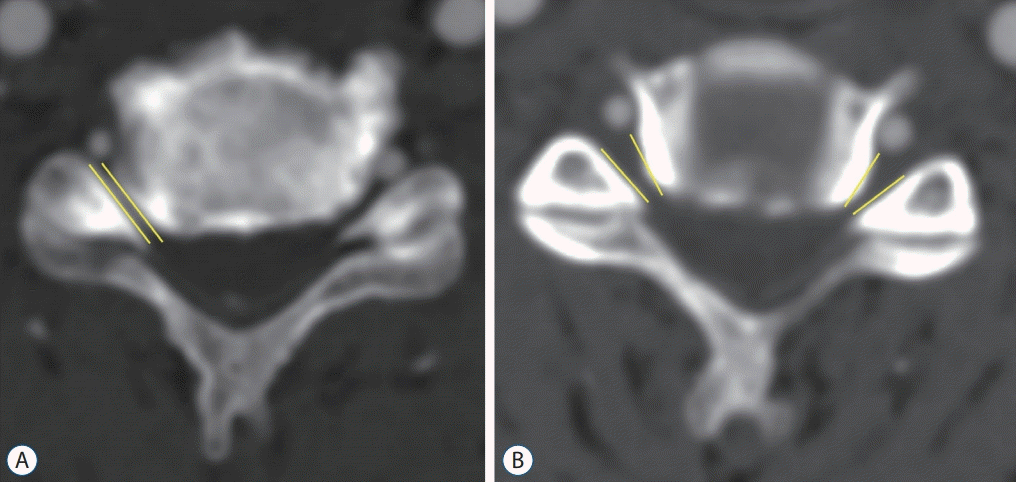
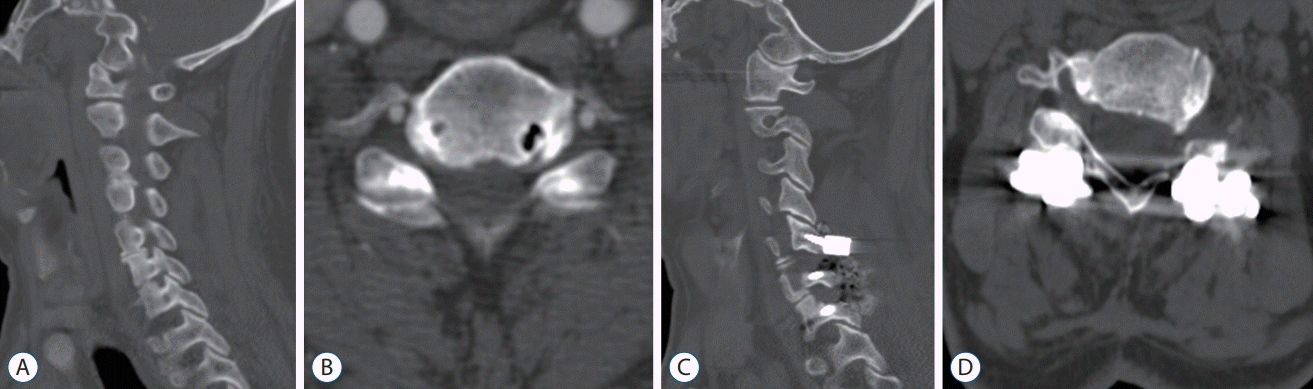
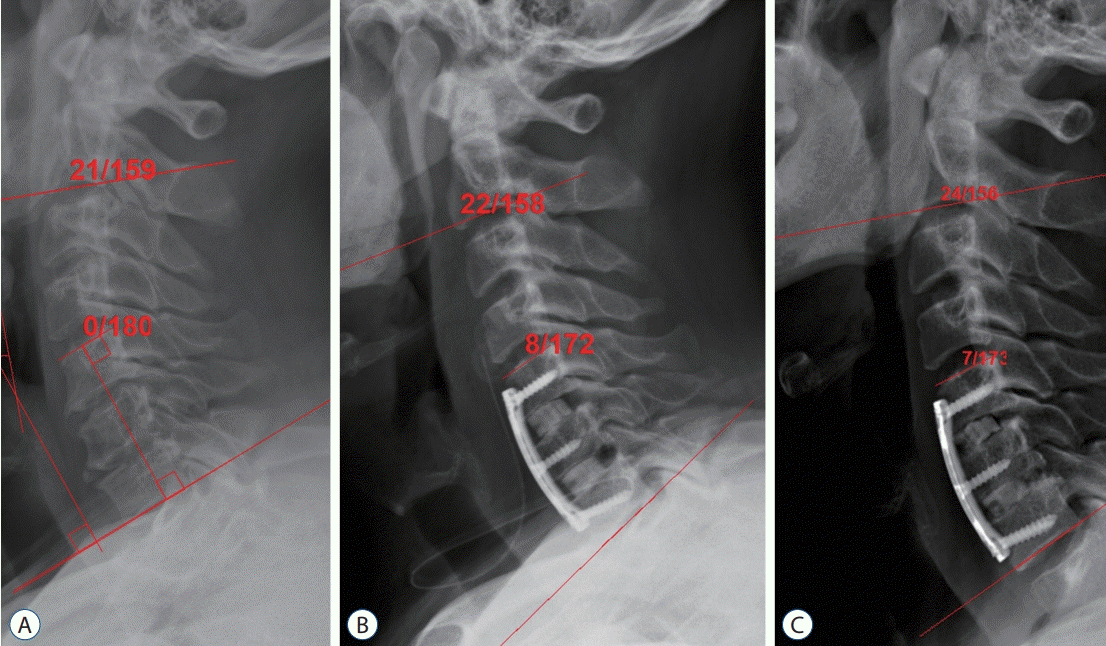
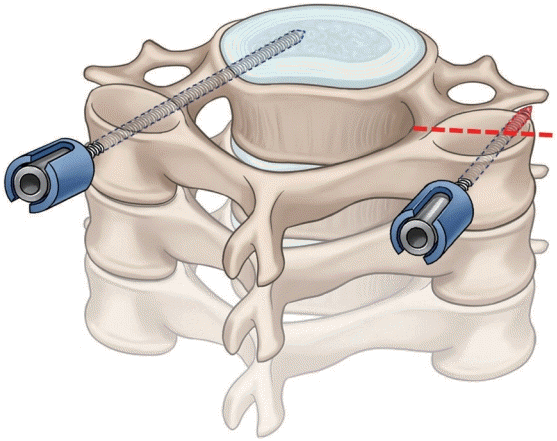




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download