1. Abi-Aad KR, Aoun RJN, Rahme RJ, Ward JD, Kniss J, Kwasny MJ, et al. New generation hydrogel endovascular aneurysm treatment trial (HEAT): a study protocol for a multicenter randomized controlled trial. Neuroradiology. 60:1075–1084. 2018.

2. Bescós JO, Slob MJ, Slump CH, Sluzewski M, van Rooij WJ. Volume measurement of intracranial aneurysms from 3D rotational angiography: improvement of accuracy by gradient edge detection. AJNR Am J Neuroradiol. 26:2569–2572. 2005.
3. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1:307–310. 1986.

4. Can A, Ho AL, Emmer BJ, Dammers R, Dirven CM, Du R. Association between vascular anatomy and posterior communicating artery aneurysms. World Neurosurg. 84:1251–1255. 2015.

5. Chan SH, Wong KS, Woo YM, Chan KY, Leung KM. Volume measurement of the intracranial aneurysm: a discussion and comparison of the alternatives to manual segmentation. J Cerebrovasc Endovasc Neurosurg. 16:358–363. 2014.

6. Costalat V, Maldonado IL, Strauss O, Bonafé A. Toward accurate volumetry of brain aneurysms: combination of an algorithm for automatic thresholding with a 3D eraser tool. J Neurosci Methods. 198:294–300. 2011.

7. Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui AH, et al. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery. 63:185–196. discussion 196-197. 2008.

8. Escobar-de la Garma VH, Zenteno M, Padilla-Vázquez F, San-Juan D, Cerón-Morales A. Comparative analysis of aneurysm volume by different methods based on angiography and computed tomography angiography. Neurosurg Rev. 41:1013–1019. 2018.

9. Fanning NF, O’Dwyer HM, Bowden JA, Brennan PR, Thornton J. Accuracy of voxel-based and algebraic formula-based methods in quantifying cerebral aneurysm volume by 3D-rotational digital subtraction angiography. An in-vitro and in-vivo study. Interv Neuroradiol. 11:35–40. 2005.

10. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. 30:1323–1341. 2012.

11. Ferroli P, Tringali G, Acerbi F, Schiariti M, Broggi M, Aquino D, et al. Advanced 3-dimensional planning in neurosurgery. Neurosurgery 72 Suppl. 1:54–62. 2013.

12. Han Q, Zhao X, Wang C, Chen B, Wang X, Zhang Z, et al. Individualized reconstruction for severe periprosthetic fractures around the tumor prosthesis of knee under assistance of 3D printing technology: a case report. Medicine (Baltimore). 97:e12726. 2018.
13. Ho AL, Mouminah A, Du R. Posterior cerebral artery angle and the rupture of basilar tip aneurysms. PLoS One. 9:e110946. 2014.

14. Jiang H, Shen J, Weng YX, Pan JW, Yu JB, Wan ZA, et al. Morphology parameters for mirror posterior communicating artery aneurysm rupture risk assessment. Neurol Med Chir (Tokyo). 55:498–504. 2015.

15. Jiang H, Weng YX, Zhu Y, Shen J, Pan JW, Zhan RY. Patient and aneurysm characteristics associated with rupture risk of multiple intracranial aneurysms in the anterior circulation system. Acta Neurochir (Wien). 158:1367–1375. 2016.

16. Kawanabe Y, Sadato A, Taki W, Hashimoto N. Endovascular occlusion of intracranial aneurysms with Guglielmi detachable coils: correlation between coil packing density and coil compaction. Acta Neurochir (Wien). 143:451–455. 2001.

17. Leng B, Zheng Y, Ren J, Xu Q, Tian Y, Xu F. Endovascular treatment of intracranial aneurysms with detachable coils: correlation between aneurysm volume, packing, and angiographic recurrence. J Neurointerv Surg. 6:595–599. 2014.

18. Lin N, Ho A, Charoenvimolphan N, Frerichs KU, Day AL, Du R. Analysis of morphological parameters to differentiate rupture status in anterior communicating artery aneurysms. PLoS One. 8:e79635. 2013.

19. Lin N, Ho A, Gross BA, Pieper S, Frerichs KU, Day AL, et al. Differences in simple morphological variables in ruptured and unruptured middle cerebral artery aneurysms. J Neurosurg. 117:913–919. 2012.

20. Munkvold BKR, Bø HK, Jakola AS, Reinertsen I, Berntsen EM, Unsgård G, et al. Tumor volume assessment in low-grade gliomas: a comparison of preoperative magnetic resonance imaging to coregistered intraoperative 3-dimensional ultrasound recordings. Neurosurgery. 83:288–296. 2018.

21. Murayama Y, Viñuela F, Ishii A, Nien YL, Yuki I, Duckwiler G, et al. Initial clinical experience with matrix detachable coils for the treatment of intracranial aneurysms. J Neurosurg. 105:192–199. 2006.

22. Otani T, Nakamura M, Fujinaka T, Hirata M, Kuroda J, Shibano K, et al. Computational fluid dynamics of blood flow in coil-embolized aneurysms: effect of packing density on flow stagnation in an idealized geometry. Med Biol Eng Comput. 51:901–910. 2013.

23. Piotin M, Daghman B, Mounayer C, Spelle L, Moret J. Ellipsoid approximation versus 3D rotational angiography in the volumetric assessment of intracranial aneurysms. AJNR Am J Neuroradiol. 27:839–842. 2006.
24. Reul J, Spetzger U, Weis J, Sure U, Gilsbach JM, Thron A. Endovascular occlusion of experimental aneurysms with detachable coils: influence of packing density and perioperative anticoagulation. Neurosurgery. 41:1160–1165. discussion 1165-1168. 1997.

25. Sadato A, Hayakawa M, Adachi K, Nakahara I, Hirose Y. Large residual volume, not low packing density, is the most influential risk factor for recanalization after coil embolization of cerebral aneurysms. PLoS One. 11:e0155062. 2016.

26. Sadato A, Hayakawa M, Tanaka T, Hirose Y. Comparison of cerebral aneurysm volumes as determined by digitally measured 3D rotational angiography and approximation from three diameters. Interv Neuroradiol. 17:154–158. 2011.

27. Satoh K, Matsubara S, Hondoh H, Nagahiro S. Intracranial aneurysm embolization using interlocking detachable coils. Correlation between volume embolization rate and coil compaction. Interv Neuroradiol 3 Suppl. 2:125–128. 1997.
28. Sluzewski M, van Rooij WJ, Slob MJ, Bescós JO, Slump CH, Wijnalda D. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology. 231:653–658. 2004.

29. Sokolowski JD, Ilyas A, Buell TJ, Taylor DG, Chen CJ, Ding D, et al. SMART coils for intracranial aneurysm embolization: follow-up outcomes. J Clin Neurosci. 59:93–97. 2019.

30. Stapleton CJ, Torok CM, Rabinov JD, Walcott BP, Mascitelli JR, Leslie-Mazwi TM, et al. Validation of the modified Raymond-Roy classification for intracranial aneurysms treated with coil embolization. J Neurointerv Surg. 8:927–933. 2016.

31. Strik HM, Borchert H, Fels C, Knauth M, Rienhoff O, Bähr M, et al. Three-dimensional reconstruction and volumetry of intracranial haemorrhage and its mass effect. Neuroradiology. 47:417–424. 2005.

32. Takao H, Ishibashi T, Saguchi T, Arakawa H, Ebara M, Irie K, et al. Validation and initial application of a semiautomatic aneurysm measurement software: a tool for assessing volumetric packing attenuation. AJNR Am J Neuroradiol. 35:721–726. 2014.

33. Wakhloo AK, Gounis MJ, Sandhu JS, Akkawi N, Schenck AE, Linfante I. Complex-shaped platinum coils for brain aneurysms: higher packing density, improved biomechanical stability, and midterm angiographic outcome. AJNR Am J Neuroradiol. 28:1395–1400. 2007.

34. Zhao R, Shen J, Huang QH, Nie JH, Xu Y, Hong B, et al. Endovascular treatment of ruptured tiny, wide-necked posterior communicating artery aneurysms using a modified stent-assisted coiling technique. J Clin Neurosci. 20:1377–1381. 2013.

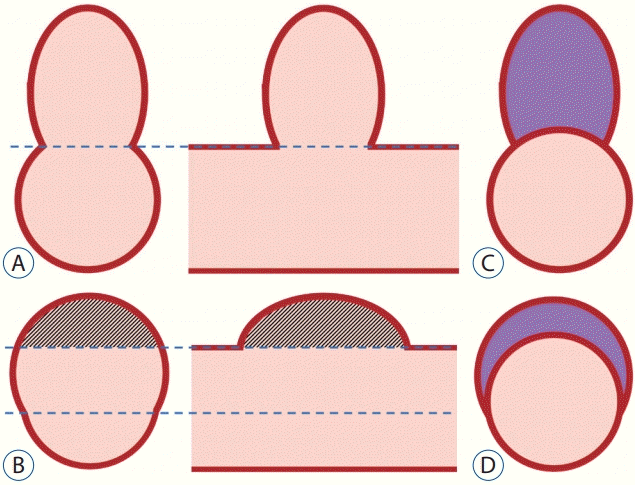
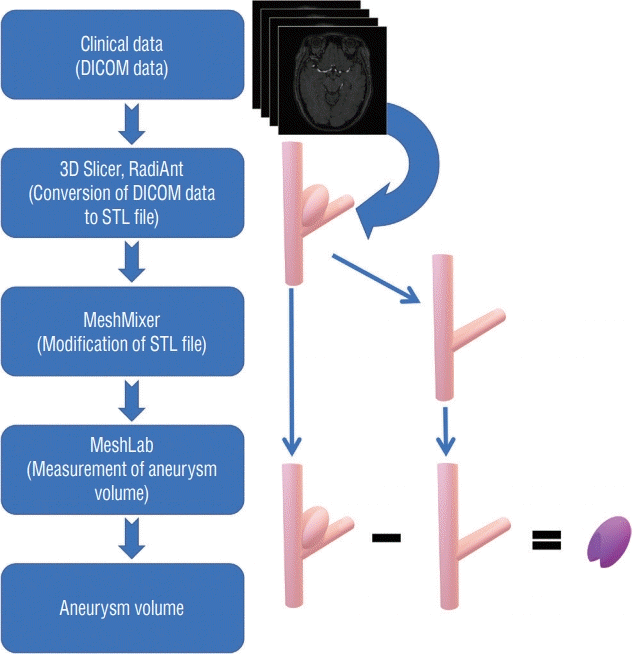
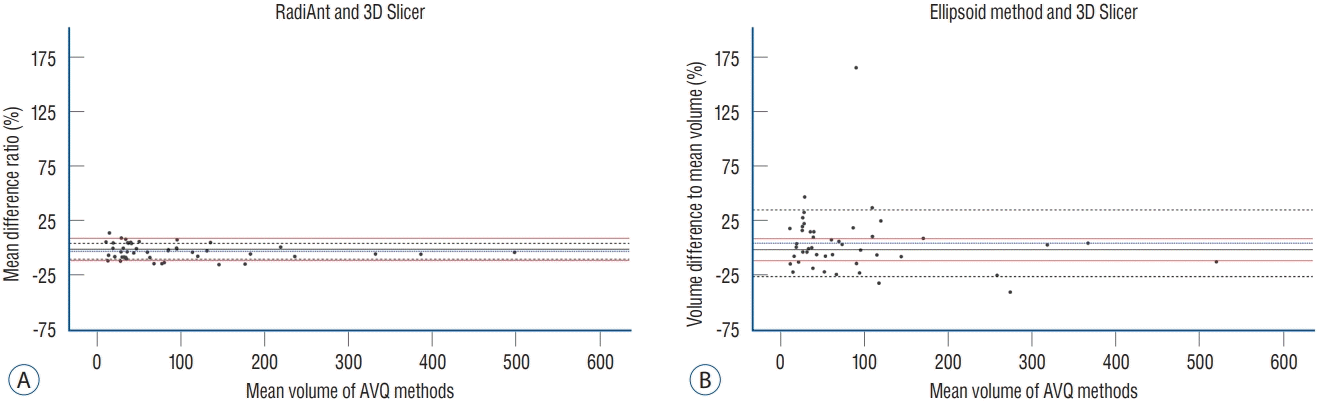
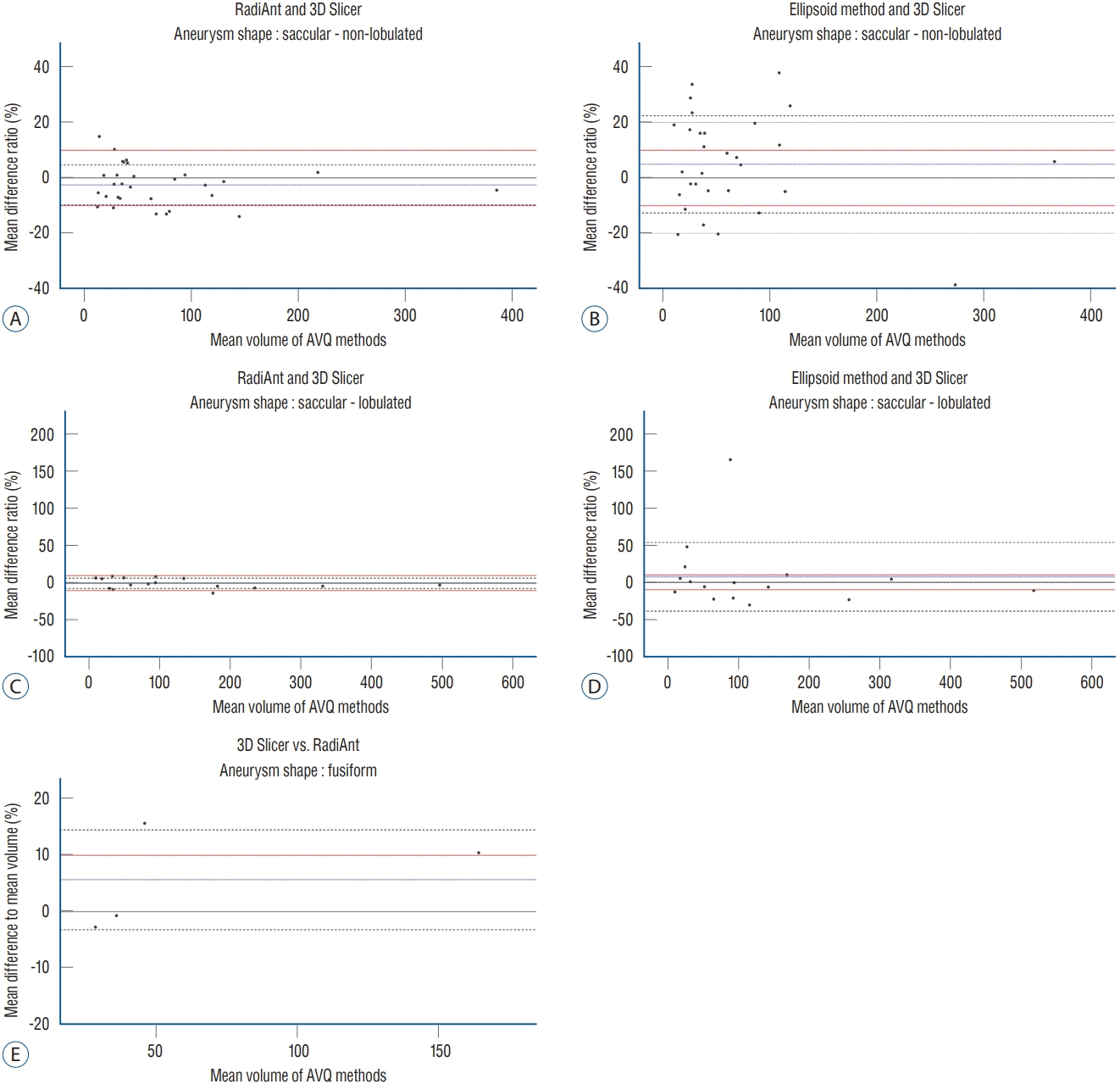




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download