1. Almallouhi E, Al Kasab S, Sattur MG, Lena J, Jabbour PM, Sweid A, et al. Incorporation of transradial approach in neuroendovascular procedures: defining benchmarks for rates of complications and conversion to femoral access. J Neurointerv Surg. 12:1122–1126. 2020.

2. Beer-Furlan A, Joshi KC, Munich SA. Radial access for acute stroke thrombectomy. Endovascular Today. 19:66–68. 2020.
3. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 372:11–20. 2015.
4. Boulanger M, Lapergue B, Turjman F, Touzé E, Anxionnat R, Bracard S, et al. First-line contact aspiration vs stent-retriever thrombectomy in acute ischemic stroke patients with large-artery occlusion in the anterior circulation: systematic review and meta-analysis. Interv Neuroradiol. 25:244–253. 2019.

5. Chen SH, Snelling BM, Sur S, Shah SS, McCarthy DJ, Luther E, et al. Transradial versus transfemoral access for anterior circulation mechanical thrombectomy: comparison of technical and clinical outcomes. J Neurointerv Surg. 11:874–878. 2019.

6. Chueh JY, Kang DH, Kim BM, Gounis MJ. Role of balloon guide catheter in modern endovascular thrombectomy. J Korean Neurosurg Soc. 63:14–25. 2020.

7. Crockett MT, Selkirk GD, Chiu AHY, Singh TP, McAuliffe W, Phillips TJ. First line transradial access for posterior circulation stroke intervention; initial 12-month experience at a high volume thrombectomy center. J Clin Neurosci. 78:194–197. 2020.

8. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 387:1723–1731. 2016.

9. Haussen DC, Nogueira RG, DeSousa KG, Pafford RN, Janjua N, Ramdas KN, et al. Transradial access in acute ischemic stroke intervention. J Neurointerv Surg. 8:247–250. 2016.

10. Hess CN, Peterson ED, Neely ML, Dai D, Hillegass WB, Krucoff MW, et al. The learning curve for transradial percutaneous coronary intervention among operators in the United States: a study from the National Cardiovascular Data Registry. Circulation. 129:2277–2286. 2014.

11. Jo KW, Park SM, Kim SD, Kim SR, Baik MW, Kim YW. Is transradial cerebral angiography feasible and safe? A single center’s experience. J Korean Neurosurg Soc. 47:332–337. 2010.

12. Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 377:1409–1420. 2011.

13. Joshi KC, Beer-Furlan A, Crowley RW, Chen M, Munich SA. Transradial approach for neurointerventions: a systematic review of the literature. J Neurointerv Surg. 12:886–892. 2020.

14. Kidwell CS, Jahan R, Alger JR, Schaewe TJ, Guzy J, Starkman S, et al. Design and rationale of the mechanical retrieval and recanalization of stroke clots using embolectomy (MR RESCUE) trial. Int J Stroke. 9:110–116. 2014.

15. Kim SH, Choi JH, Kang MJ, Cha JK, Kim DH, Nah HW, et al. Efficacy of combining proximal balloon guiding catheter and distal access catheter in thrombectomy with stent retriever for anterior circulation ischemic stroke. J Korean Neurosurg Soc. 62:405–413. 2019.

16. Oselkin M, Satti SR, Sundararajan SH, Kung D, Hurst RW, Pukenas BA. Endovascular treatment for acute basilar thrombosis via a transradial approach: initial experience and future considerations. Interv Neuroradiol. 24:64–69. 2018.

17. Patel P, Haussen DC, Nogueira RG, Khandelwal P. The neuro radialist. Interv Cardiol Clin. 9:75–86. 2020.

18. Rajah GB, Lieber B, Kappel AD, Luqman AW. Distal transradial access in the anatomical snuffbox for balloon guide-assisted stentriever mechanical thrombectomy: technical note and case report. Brain Circ. 6:60–64. 2020.

19. Ribo M, Flores A, Rubiera M, Pagola J, Mendonca N, Rodriguez-Luna D, et al. Difficult catheter access to the occluded vessel during endovascular treatment of acute ischemic stroke is associated with worse clinical outcome. J Neurointerv Surg 5 Suppl. 1:i70–i73. 2013.

20. Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the multi MERCI trial. Stroke. 39:1205–1212. 2008.
21. Spiotta AM, Vargas J, Turner R, Chaudry MI, Battenhouse H, Turk AS. The golden hour of stroke intervention: effect of thrombectomy procedural time in acute ischemic stroke on outcome. J Neurointerv Surg. 6:511–516. 2014.

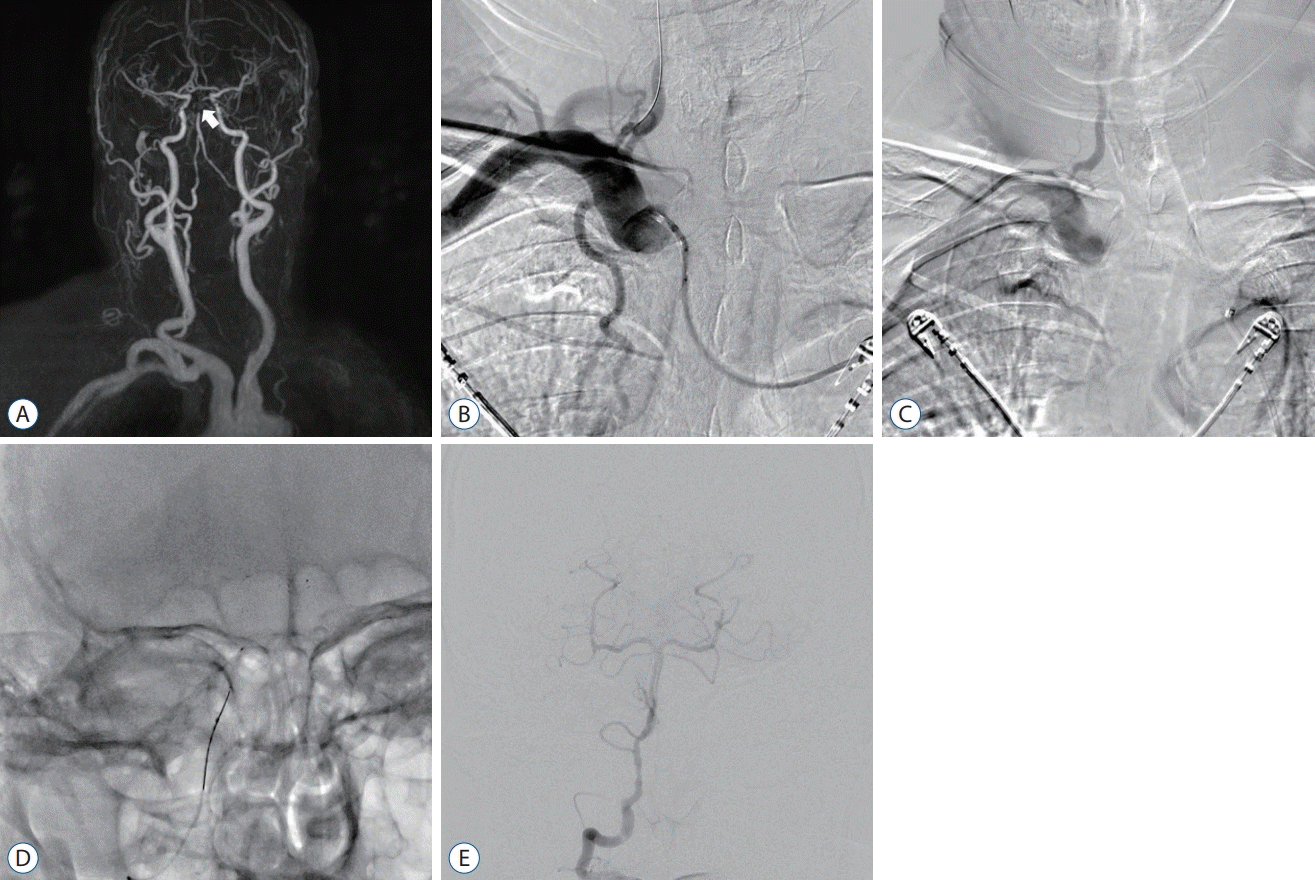
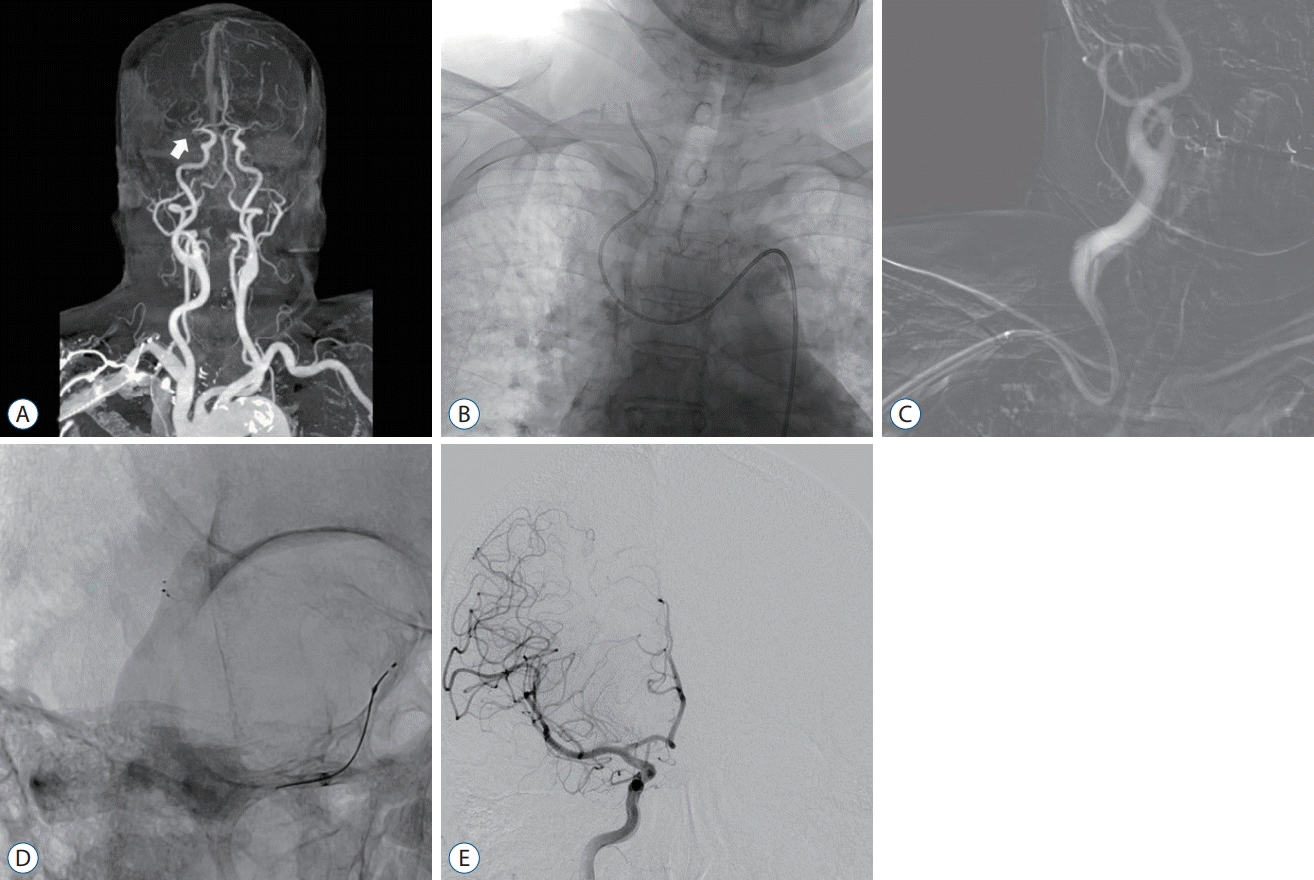




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download