INTRODUCTION
CASE REPORT
Case 1
History and examination
Operation
Postoperative course
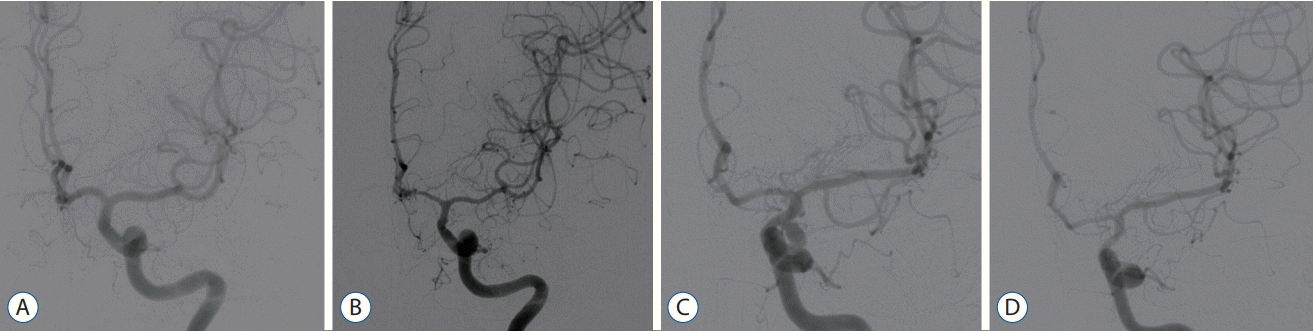 | Fig. 1.A : Preoperative angiography of case 1. b : Postoperative angiography of case 1 presenting significant cerebral vasospasm on the left middle cerebral artery and A1. c : Preoperative angiography of case 2. d : Postoperative angiography of case 2 presenting significant cerebral vasospasm on left A1 and M1 segment. |
Case 2
History and examination
Operation
Postoperative course
DISCUSSION
Table 1.
| Study | Sex/age | Aneurysm location | Aneurysm size | Neurologic deficit | POD | Intra op event | Postoperative hemorrhage | Location of vasospasm | Treatment | Recovery |
|---|---|---|---|---|---|---|---|---|---|---|
| Bloomfield and Sonntag [3] (1985) | F/54 | Rt. MCA bifurcation | 7 mm | Lt. side weakness Gr. III | 9 | When clipped An. puncturing, MCA focal spasm revealed | No | Rt. ICAsupraclinoid, Rt. ACA, Rt. MCA | Hydration dexamethasone 4 mg/hr for 5 days | Partially Lt. side weakness IV |
| Gutiérrez et al. [10] (2001) | F/55 | Lt. opthalmic | 5 mm | Rt. hemiparesis, aphasia | 16 hours after | No | No | M1, M2, A1, A2 | IA papaverine M1, M2, A1, A2 | Slight Rt. hemiparesis |
| Kitazawa et al. [14] (2005) | F/21 | Lt. paraclinoid | 4 mm | Aphasia, gerstmann syndrome | 12 | No | No | Lt. M2 | Chemical angioplasty Triple H | Fully |
| Kitazawa et al. [14] (2005) | F/53 | Lt. paraclinoid | 4 mm | Aphasia | 9 | No | No | Triple H | Fully | |
| Kitazawa et al. [14] (2005) | F/63 | Lt. paraclinoid | 5 mm | Aphasia,Rt. side weakness IV | 5 | No | Mild EDH | Rt. ACA, MCA | Triple H, IA papaverine | Fully |
| Paolini et al. [17] (2005) | F/47 | Rt. MCA bifurcation | 8 mm | Lt. facial droop Lt. side weakness IV | 28 | No | Small ICH around clipping site | Rt. M1 segment | Hydration | Fully |
| Antiplatelet | ||||||||||
| Hiroaki et al. [11] (2016) | F/62 | Lt. PCoA | 5 mm | Aphagia, Rt. hemiplegia | 11 | No | No | Bilateral A1, bilateral PCA, Lt. M1, Rt. M3–M4 | Hypervolemic and antiplatelet Tx. | Acalculia and paraphasia |
| Yang et al. [20] (2015) | F/41 | Lt. ICA bifurcation | 5 mm | Aphasia Rt. facial numbness | 28 | No | No | Lt. A1, M1 | Hydration | Partially minimal aphasia |
| Antiplatelet | ||||||||||
| Chemical angioplasty | ||||||||||
| Yang et al. [20] (2015) | F/61 | Lt. MCA | 6 mm | Aphasia | 10 | No | No | - | Hydration | Partially minimal aphasia |
| Antiplatelet | ||||||||||
| Chemical angioplasty | ||||||||||
| Tsyben et al. [18] (2016) | F/53 | Lt. distal M1 | 5 mm | Dysphasia, Rt. hemiparesis predominantly upper limb | 30 hours after | No | No | Lt. MCA | IA verapamil | Minimal dysphasia right upper limb weakness |
| Tsyben et al. [18] (2016) | M/70 | ACoA, small two Lt. MCA | Right hemiparesis | 2 | No | No | Lt. M2, M3 | IA verapamil nimodipine 21 days moderate induced hypertension hypervolemia | Fully | |
| Campe et al. [4] (2019) | F/69 | Rt. MCA | - | Left facial palsy Left hemiparesis | 12 | No | No | Rt. M1, M2 | Antithrombotic Tx. with aspirin | Fully |
| Oral nimodipine | ||||||||||
| Macro et al. [16] (2020) | F/59 | Lt. MCA bifurcation | 5 mm | Rt. hemiparesis, aphasia | 6 | No | Lt. frontobasal and temporal hypodensity | Lt. M1, MCA bifurcation | IA nimodipine | Dysphasia |
| Cuoco et al. [6] (2020) | F/53 | Lt. MCA bifurcation | 5 mm | Rt. hemiparesis, hemianopsia | 13 and 26 | No | No | POD15-diffuse | Hypervolemia | Rt. Hemiphasesis hemianopsia |
| POD26-Rt. P1 | IA nimodipine |
POD : postoperative day, op : operation, F : female, Rt. : right, MCA : middle cerebral artery, Lt. : left, Gr. : grade, An. : aneurysm, ICA : internal carotid artery, ACA : anterior cerebral artery, IA : intraarterial, Triple H : hypertension, hypervolemia, and hemodilution, SAH : subarachnoid hemorrhage, EDH : epidural hemorrhage, ICH : intracerebral hemorrhage, PCoA : posterior communicating artery, PCA : posterior cerebral artery, Tx. : treatment, M : male, ACoA : anterior communicating artery




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download