Abstract
Compared to any other decade, the last two decades have been the most dynamic period in terms of advances in the knowledge on spinal dysraphism. Among the several factors of rapid advancement, such as embryology during secondary neurulation and intraoperative neurophysiological monitoring, there is no doubt that Professor Dachling Pang stood high amidst the period. I review here the last two decades from my personal point of view on what has been achieved in the field of spinal dysraphism, focusing on occult tethered cord syndrome, lumbosacral lipomatous malformation, terminal myelocystocele, retained medullary cord, limited dorsal myeloschisis and junctional neural tube defect. There are still many issues to revise, add and extend. Profound knowledge of basic science is critical, as well as refined clinical analysis. I expect that young scholars who follow the footsteps of precedent giants will shed bright light on this topic in the future.
Compared to any other decade, the last two decades have been the most dynamic period in terms of advances in the knowledge on spinal dysraphism. Among the several factors of rapid advancement, such as embryology during secondary neurulation and intraoperative neurophysiological monitoring (IONM), there is no doubt that Professor Dachling Pang stood high amidst the period.
I review here the last two decades from my personal point of view on what has been achieved in the field of spinal dysraphism. In this article, neurological deficits include neurogenic bladder and bowel as well as motor, sensory and reflex abnormalities.
In 1953, ‘filum terminale syndrome’, described by Garceau [8], introduced the concept that traction of the spinal cord may cause neurological deficits. In 1976, Hoffman et al. [9] coined the term ‘TCS’ for thick filum terminale, and it has been used for a wide variety of lesions that pull the spinal cord, leading to neurological problems. The pathophysiology of TCS proposed by Yamada et al. [29] in 1981 and Kang et al. [10] in 1987 added a more solid scientific basis to this entity.
When I started to work as a junior staff pediatric neurosurgeon in 1987, I came to realize that even in the absence of neuroradiological evidence of TCS, such as low-lying conus or thick filum, neurological deficits may be caused by TCS with normal-looking filum, and TCS may be reversed by filum section. This was ‘occult TCS’, which was described by Selçuki and Coşkun [27] later in 1998. When I stayed in the USA during the period of 1990–1992, I met a famous pediatric neurosurgeon in Boston and heard an experience in which he identified a non-tensile normal-looking filum at surgery and gave up filum section in such a patient. At that time, many neurosurgeons still hesitated to cut the filum in cases of occult TCS.
Now, the concept of occult TCS is widely accepted, and I do not hesitate to perform filum sections when the patients show typical clinical findings : 1) normal neuroimaging findings of the conus and filum and 2) new or progressive neurological deficits (mainly urinary incontinence) or pain.
When I was a resident (1980–1984), the concepts of the entity known as ‘LLM’ were not well established, and the surgical technique was not well developed. Since a history-making article by Chapman [4] in 1982, remarkable progress has been made in the management of LLM. When I started my career as a pediatric neurosurgeon in 1987, there was a firm belief that all LLM lesions must be operated on for the prevention of progressive neurological deficits, and the standard procedure was untethering and partial removal of the fat. Radical removal was believed to be associated with spinal cord injury. I read an article that was published in 1957 showing five cases in which intramedullary lipomas were totally removed with good results [3]. However, partial or subtotal removal was the mainstream treatment in the management of LLM, even in their article. Without any discomfort, I was doing the standard procedure: complete untethering, partial removal of fat and pial reconstruction with or without duraplasty.
It was in 1997 when Pierre-Kahn et al. [26] published a historical article reporting worse long-term outcome in the group of patients with operated asymptomatic conus LLM than in the unoperated group. I was shocked and confused on the medical advice for these patients. I explained the changes in the experts’ opinion and gave the parents both options, operation or observation, if the patients were asymptomatic. Nevertheless, many of the parents chose surgery because of fear that they may miss the appearance of symptoms. In 2009 and 2010, other historical articles were published by Pang et al. [23,24] They showed the short-term and long-term favorable outcomes in the group operated on by radical fat removal and emphasized the long-term prognostic value of reducing the cord/dural sac ratio by the analysis of a large number of patients. The advancement of IONM made this aggressive surgery possible. Currently, although surgical indication may vary according to surgeons, radical fat removal is widely accepted as a standard method of LLM surgery when surgery is decided. It reminded me of the five cases of total removal of intramedullary lipomas with good outcome in that old article [3]. I hope many other groups publish reports reproducing the data of Pang et al. [24] in the near future.
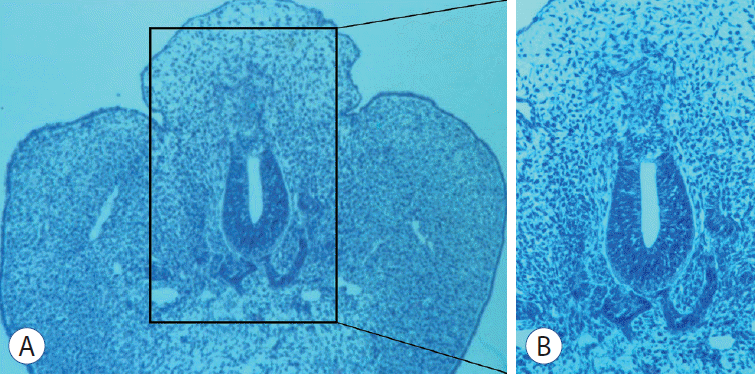
In terms of the pathoembryogenesis of dorsal-type LLM, I have tried to test the ‘premature disjunction’ theory proposed by McLone and Naidich [15] and Naidich et al. [17] using chick embryos. McLone and Naidich [15] explained the occurrence of dorsal-type LLM by premature disjunction of the unilateral neural fold into discontinued neuroectoderm and surface ectoderm. This event permits an invasion of the surrounding mesenchymal tissue into the neural tube through the gap between the two ectoderms during the process of neural tube formation (closure). I made incisions on the unilateral distal neural fold with a fine needle and obtained specimens showing findings of LLM (Fig. 1) [14]. These results support the ‘premature disjunction’ theory.
When I stayed at Children’s Memorial Hospital in Chicago from 1990 to 1992 as a research fellow, I heard of TMCC, an entity newly proposed by McLone and Naidich [16] in 1985. They described the characteristics of the lesion and suggested its pathoembryogenesis, a ‘ballooned terminal ventricle’. They suggested the entrapment of cerebrospinal fluid in the neural tube, the dilatation of the terminal ventricle, and subsequent disruption of the dorsal mesenchyme by the dilated terminal ventricle, resulting in direct contact of the neural tube with the subcutaneous layers of the superficial ectoderm.
When I came back to Korea, I started laboratory research on open neural tube defects (NTDs) using chick embryos. Soon, the incidence of open NTDs rapidly declined in Korea, and my interest went to secondary neurulation. At that time, the knowledge of secondary neurulation was quite limited among clinicians, including pediatric pathologists. I investigated the whole process of secondary neurulation in chick embryos by light microscopic findings and published the data in 2003 [31]. One of the interesting findings that I found at that time is the ‘transient terminal balloon’ of the secondary neural tube, which directly contacts the cutaneous ectoderm with intact basement membranes between them. I thought that the persistence of this connection may be the reason for TMCC. In my embryological lectures, I showed this terminal balloon and suggested it as the cause of TMCC.
Around 2011, Professor Pang sent me an e-mail saying he found an interesting article explaining the pathoembryogenesis of TMCC while he was sitting on a jury. It was the article that my team published in 2003. Through that communication, Dr. Pang’s group and my team wrote an article on the clinical and embryological aspects of TMCC in 2012 [21]. My team examined detailed morphological processes in the formation and regression of terminal balloons using chick embryos and published the data in 2013 [12]. Despite species differences, these articles shed light on the pathoembryogenesis of TMCC. Although limited in the number of specimens, my team found similar findings of the terminal balloon in normal human embryos thereafter [30].
From the time when I became a staff pediatric neurosurgeon in 1987, I have seen many cases of low-lying conus. At that time, I routinely cut the filum. Some of them had a very low-lying conus ending at the cul-de-sac. Some had cysts, whereas some had fatty components. Those lesions were called TCS, and when filum could be identified, we also called them ‘thickened filum’ or ‘tethered spinal cord in a strict sense’. We knew that the lesions were the result of failed regression in the normal process of secondary neurulation.
In some of these cases, there is functionless spinal cord tissue at the end of the conus that is a remnant of the medullary cord caused by ‘failed regression’. For those lesions, Pang et al. [22] coined the term ‘RMC’. Previously, I had not confirmed the presence of a nonfunctional end of the conus by IONM. I began to cut the nonfunctional tip of the RMC if the nonfunctional tip was located very low (close to the cul-de-sac) or was cystic to make a long gap between the upper cut end of the RMC and the cul-de-sac for the prevention of retethering. If the tip of the non-cystic ‘conus’ is located well above the cul-de-sac, I do not routinely check for the presence of a nonfunctional tip, and I just cut the filum to save the length of laminectomy. In that case, the diagnosis of RMC cannot be confirmed by IONM.
The clinical significance of congenital dermal sinus (CDS) is well known: infection, mass effect by associated tumors and chemical inflammation by the leakage of tumor contents. I have found that not a small number of patients, however, show similar neuroimaging findings as those with CDS but have different skin lesions (frequently cigarette-burn scars). The squamous epithelial components were absent in the stalk. In 2010, Pang et al. [25] coined the term ‘LDM’, a failure of normal disjunction of the neuroectoderm from the surface ectoderm, pulling the neural tissue to the skin side. If the skin is pulled to the neural side, CDS is the result. They classified the lesions into flat (non-cystic) and cystic types. Recently, my team reported that LDM may be found in the area of secondary neurulation (Fig. 2) [11]. The continuation of the caudal cell mass with the surface-located primitive streak in the early phase of secondary neurulation gives the chance for non-disjunction to occur.
The introduction of the concept of LDM is another solution of long-lasting enigmas. Previously, the lesions were called ‘tethered spinal cord by a stalk which is connected to the back skin’, ‘skin-covered myelomeningocele’, ‘spinal meningocele with neural tissue in the wall’, ‘cervical myelomeningocele’ or ‘myelocystocele’.
LDM, both flat and cystic types, can be combined with CDS [13]. The term ‘limited dorsal (or ’focal’) non-disjunctional disorders’, which was also used in this Pediatric Issue, includes LDM, CDS, and the mixed form of the two entities. All three lesions are in the same spectrum.
When I visited Oakland in 2009, Professor Pang showed me a patient with a JNTD who had a morphologically and functionally isolated conus. He asked me, “Which is closer to human in terms of secondary neurulation, mouse or chick?” I answered “chick.” I thought that the late joining of the lumens of the primary and secondary neural tubes in chick embryos seemed closer to that of human than mouse embryos. Both of us agreed on ‘chick’.
In 2017, his team introduced the concept of JNTD [7]. He explained the pathoembryogenesis by failed connection between the primary and secondary neural tubes, which is caused by mutation of planar cell polarity pathway genes. The description of the normal process of junctional neurulation by Dady et al. [5] led to the concept of JNTD.
My team published an article suggesting that segmental spinal dysgenesis (SSD) is a continuum of JNTD [28]. SSD has a functionally separated conus-like JNTD, but the lesion may involve the lower thoracic spinal cord and is associated with bony stenosis at the level of a band connecting ‘upper’ and ‘lower’ spinal cord segments. The tracer study of Dady et al. [5] showed that the overlapping zone of junctional neurulation extends up to the low thoracic spinal cord in humans. It also matches well with the involvement of the lower thoracic spinal cord in some cases of caudal agenesis, another disorder of secondary neurulation. However, the relationship between JNTD and SSD is a subject of controversy.
Before the last two decades, theories of the pathoembryogenesis of split cord malformation (SCM; diastematomyelia, diplomyelia) were proposed. They included split notochord theory and accessory neurenteric canal theory [1,2,6,20]. The former assumed an abnormal endoderm-ectodermal adhesion at the midline. The notochordal process growing cephalad is divided into two hemi-notochordal processes and induces two neural tubes. The adhered endoderm and ectoderm with adjacent mesenchymal tissue may induce various types of tissue in the space between the two neural tubes. Some researchers regarded abnormal double notochord formation as an abnormality of notochordal tissue or Hensen’s node itself, not the result of endoderm-ectoderm midline adhesion or a traversing structure. In the accessory neurenteric canal theory, the neurenteric canal that traverses from the amniotic cavity to the yolk sac (umbilical vesicle) plays the role of endoderm-ectoderm adhesion in split notochord theory. The traversing structure from the ectoderm to the endoderm and surrounding mesenchymal tissue may induce various types of tissue from the three germ layers.
In 1992, Pang [18] and Pang et al. [20] described the pathoembryogenesis of SCM with its associated anomalies. They divided SCM into types I and II according to the separation of the dural sac into two or not and emphasized the presence of tethering bands and necessity of untethering of the bands in cases of SCM type II.
Before the last two decades, Professor Pang described the entity of sacral agenesis in detail [19]. Although the definition is abnormalities of bone, it has clinical significance in the aspects of neural function and multi-organ formation. Until now, the article stands almost alone in terms of caudal agenesis.
Recently, with the request of writing a chapter in the textbook of the International Society for Pediatric Neurosurgery from Professor Pang, my team depicted the clinical features of caudal agenesis with correlation to embryology in more detail. The association of multi-organ anomalies was explained by the abnormal weak proliferation capacity of caudal mesenchymal tissues during the process of secondary neurulation. The neurological manifestations were classified into the ‘failure of formation’ and the ‘failure of regression’.
There are at least three factors that have brought rapid advancement in the field of spinal dysraphism.
The most important factor is basic research on normal neuroembryology and related molecular genetic events. Basic research provided a great momentum for understanding spinal dysraphism. It is a pride of my laboratory team that we could contribute to this basic research. My team is so happy to see our slides of chick embryos, which were used during Professor Pang’s lectures.
The second factor is IONM. IONM allows us to understand the pathophysiology of the lesions and to perform more radical operations with improved outcomes. IONM is especially critical for the diagnosis and treatment of LLM, TMCC, RMC, and JNTD.
The third factor is outstanding scholars such as Professor Pang. Their genius and tenacious research during the last two decades led to a new era of rapid advancement.
Professor Pang is a pioneer not just for the field of spinal dysraphism but also for many other fields, including spinal cord injury without radiological abnormalities (SCIWORA), atlanto-axial rotatory subluxation, SCM, craniovertebral junction anomalies and low-pressure hydrocephalus. He introduced new concepts and coined many new terms. He is a workaholic in academic research and publication as well as in clinical practice. The variety of books he reads is vast. He likes books on humanities, and his bookshelves are full of old classical publications. He also has profound knowledge of classical music. Every weekend, he sits at a small coffee shop with his wife Lynne, a former TV news anchor, and reads articles or writes manuscripts. Moreover, he has great talent in drawing. The artistic drawings inserted in his articles look like celebrated paintings in the museum. He is a great supporter for Korean friends (Fig. 3).
There are still many issues to revise, add, and extend. Some entities still have controversies. Profound knowledge of basic science is critical, as well as refined clinical analysis. In the decades to come, advances will continue in spinal dysraphism and its closely related fields of science and technology. I expect that young scholars who follow the role of Professor Pang will shed bright light on this topic in the future.
ACKNOWLEDGMENTS
The author expresses his sincere gratitude to Dr. Kyung
Hyun Kim for the editorial assistance.
References
1. Beardmore HE, Wiglesworth FW. Vertebral anomalies and alimentary duplications; clinical and embryological aspects. Pediatr Clin North Am. 5:457–474. 1958.

2. Bremer JL. Dorsal intestinal fistula; accessory neurenteric canal; diastematomyelia. AMA Arch Pathol. 54:132–138. 1952.
3. Caram PC, Carton CA, Scarcella G. Intradural lipomas of the spinal cord; with particular emphasis on the intramedullary lipomas. J Neurosurg. 14:28–42. 1957.
4. Chapman PH. Congenital intraspinal lipomas: anatomic considerations and surgical treatment. Childs Brain. 9:37–47. 1982.
5. Dady A, Havis E, Escriou V, Catala M, Duband JL. Junctional neurulation: a unique developmental program shaping a discrete region of the spinal cord highly susceptible to neural tube defects. J Neurosci. 34:13208–13221. 2014.

6. Dias MS, Walker ML. The embryogenesis of complex dysraphic malformations: a disorder of gastrulation? Pediatr Neurosurg. 18:229–253. 1992.

7. Eibach S, Moes G, Hou YJ, Zovickian J, Pang D. Unjoined primary and secondary neural tubes: junctional neural tube defect, a new form of spinal dysraphism caused by disturbance of junctional neurulation. Childs Nerv Syst. 33:1633–1647. 2017.

8. Garceau GJ. The filum terminale syndrome (the cord-traction syndrome). J Bone Joint Surg Am. 35-A:711–716. 1953.
9. Hoffman HJ, Hendrick EB, Humphreys RP. The tethered spinal cord: its protean manifestations, diagnosis and surgical correction. Childs Brain. 2:145–155. 1976.

10. Kang JK, Kim MC, Kim DS, Song JU. Effects of tethering on regional spinal cord blood flow and sensory-evoked potentials in growing cats. Childs Nerv Syst. 3:35–39. 1987.

11. Kim JW, Wang KC, Chong S, Kim SK, Lee JY. Limited dorsal myeloschisis: reconsideration of its embryological origin. Neurosurgery. 86:93–100. 2020.

12. Lee JY, Kim SP, Kim SW, Park S-H, Choi JW, Phi JH, et al. Pathoembryogenesis of terminal myelocystocele: terminal balloon in secondary neurulation of the chick embryo. Childs Nerv Syst. 29:1683–1688. 2013.

13. Lee JY, Park SH, Chong S, Phi JH, Kim SK, Cho BK, et al. Congenital dermal sinus and limited dorsal myeloschisis: “spectrum disorders” of incomplete dysjuction between cutaneous and neural ectoderms. Neurosurgery. 84:428–434. 2019.

14. Li YC, Shin SH, Cho BK, Lee MS, Lee YJ, Hong SK, et al. Pathogenesis of lumbosacral lipoma: a test of the “premature dysjunction” theory. Pediatr Neurosurg. 34:124–130. 2001.

15. McLone DG, Naidich TP. Spinal dysraphism : Experimental and clinical. In : Holtzman RN, Stein BM, editors. The tethered spinal cord. New York: Thieme-Stratton;1985. p. 14–28.
17. Naidich TP, McLone DG, Mutluer S. A new understanding of dorsal dysraphism with lipoma (lipomyeloschisis): radiologic evaluation and surgical correction. AJR Am J Roentgenol. 140:1065–1078. 1983.

18. Pang D. Split cord malformation: part II: clinical syndrome. Neurosurgery. 31:481–500. 1992.
19. Pang D. Sacral agenesis and caudal spinal cord malformations. Neurosurgery. 32:755–778. discussion 778-779. 1993.

20. Pang D, Dias MS, Ahab-Barmada M. Split cord malformation: part I: a unified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 31:451–480. 1992.
21. Pang D, Zovickian J, Lee JY, Moes GS, Wang KC. Terminal myelocystocele: surgical observations and theory of embryogenesis. Neurosurgery. 70:1383–1404. discussion 1404-1405. 2012.
22. Pang D, Zovickian J, Moes GS. Retained medullary cord in humans: late arrest of secondary neurulation. Neurosurgery. 68:1500–1519. discussion 1519. 2011.

23. Pang D, Zovickian J, Oviedo A. Long-term outcome of total and near-total resection of spinal cord lipomas and radical reconstruction of the neural placode: part I-surgical technique. Neurosurgery. 65:511–528. discussion 528-529. 2009.
24. Pang D, Zovickian J, Oviedo A. Long-term outcome of total and near-total resection of spinal cord lipomas and radical reconstruction of the neural placode, part II: outcome analysis and preoperative profiling. Neurosurgery. 66:253–272. discussion 272-273. 2010.
25. Pang D, Zovickian J, Oviedo A, Moes GS. Limited dorsal myeloschisis: a distinctive clinicopathological entity. Neurosurgery. 67:1555–1579. discussion 1579-1580. 2010.

26. Pierre-Kahn A, Zerah M, Renier D, Cinalli G, Sainte-Rose C, LellouchTubiana A, et al. Congenital lumbosacral lipomas. Childs Nerv Syst. 13:298–334. discussion 335. 1997.

27. Selçuki M, Coşkun K. Management of tight filum terminale syndrome with special emphasis on normal level conus medullaris (NLCM). Surg Neurol. 50:318–322. discussion 322. 1998.

28. Wang KC, Lee JS, Kim K, Im YJ, Park K, Kim KH, et al. Do junctional neural tube defect and segmental spinal dysgenesis have the same pathoembryological background? Childs Nerv Syst. 36:241–250. 2020.

29. Yamada S, Zinke DE, Sanders D. Pathophysiology of “tethered cord syndrome”. J Neurosurg. 54:494–503. 1981.

Fig. 1.
An embryo with histological findings suggestive of lumbosacral lipomatous malformation. B : A magnified view of (A). The embryo shows a back hump, a blending of neuroepithelial and mesenchymal tissues, an indistinct basement membrane of the neuroepithelium and an abnormal shape of the notochord (A : HE, ×100; B : HE, ×200). Modified from Li et al. [14] with permission from Karger Publishers, Basel, Switzerland).




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download