INTRODUCTION
MATERIALS AND METHODS
Animal model of ischemic stroke
Experimental groups
Neurobehavioral tests
Quantification and statistics
RESULTS
Effects of MFG-E8 on neurobehavioral outcomes
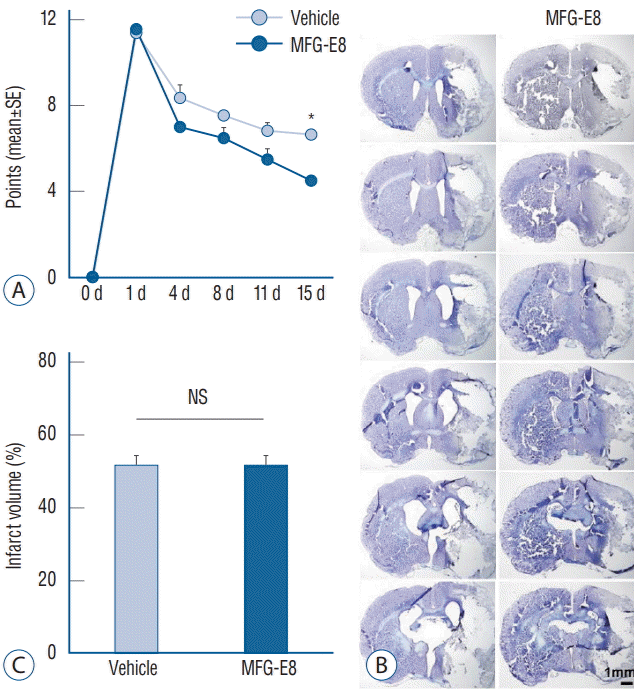 | Fig. 1.Modified Neurological Severity Scores and infarct volumes. A and C : Quantification of neurological scores and infarct volumes of vehicle-and MFG-E8-treated rats after transient middle cerebral artery occlusion (tMCAO). Neurobehavioral function improved in the MFG-E8-treated group on day 15 after tMCAO. No significant differences in percentage infarct volumes were found between groups. B : Representative images of cresyl violet-stained coronal brain sections 15 days after tMCAO. Scale bar=1 mm. *p<0.001, n=4–5 per group. SE : standard error, MFG-E8 : milk fat globule-epidermal growth factor VIII, NS : no significance. |
Cerebral infarction volume
Immunohistochemical analyses
MFG-E8 inhibits microglial activation in ischemic hemispheres
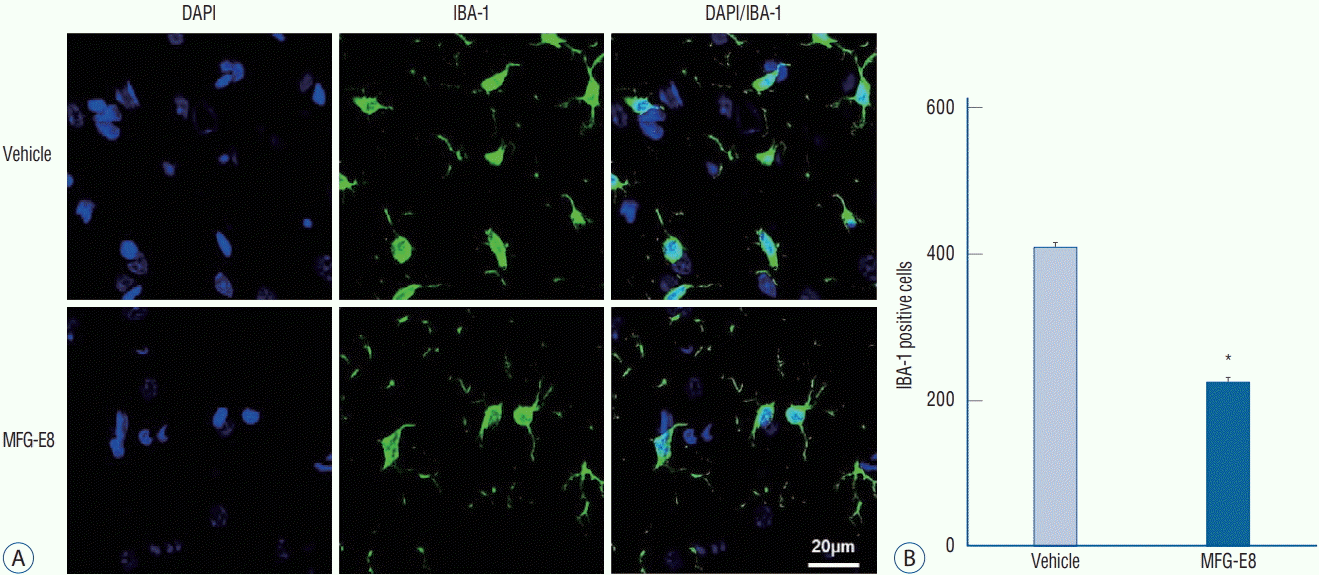 | Fig. 2.MFG-E8 inhibits microglial activation. A : Representative images of Iba-1 immunoreactivity counterstained with DAPI evaluated in ischemic hemispheres on day 15 after ischemia induction. Scale bar=20 mm. B : Quantification of the number of microglia found in the peri-infarct area. Iba1-positive cells (green) decreased in the MFG-E8-treated group compared with the vehicle-treated group. Data are expressed as mean±SEM. *p<0.001, compared with the vehicle-treated group by Student’s t-test. DAPI : 4',6-diamidino-2-phenylindole, Iba-1 : ionized calcium-binding adapter molecule 1, MFG-E8 : milk fat globule-epidermal growth factor VIII, SEM : standard error of mean. |
MFG-E8 enhances angiogenesis in the peri-infarct area in the rat brain
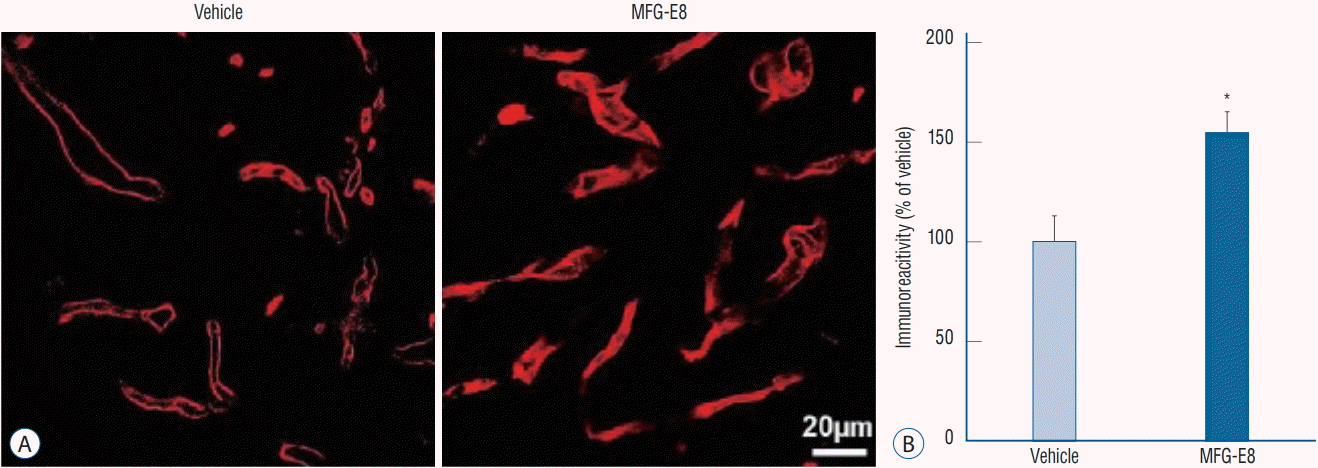 | Fig. 3.MFG-E8 enhances angiogenesis in the peri-infarct area in ischemic rat brains. A : Representative images of RECA-1-positive microvessels in ischemic hemispheres on day 15 after ischemia induction. Scale bar=20 mm. B : Quantitative analysis of RECA-1 immunoreactivity determined from 6 cryosections (n=4–5 per group). RECA-1 (a marker of endothelial cells) immunostaining shows an increased area of vessels in the MFG-E8-treated group. Data are expressed as percentages of immunoreactivity in MFG-E8-treated stroke brains compared with vehicle-treated stroke brains. *p<0.05, compared with the vehicle-treated group by Student’s t-test. MFG-E8 : milk fat globule-epidermal growth factor VIII, RECA-1 : rat endothelial cell antigen-1. |
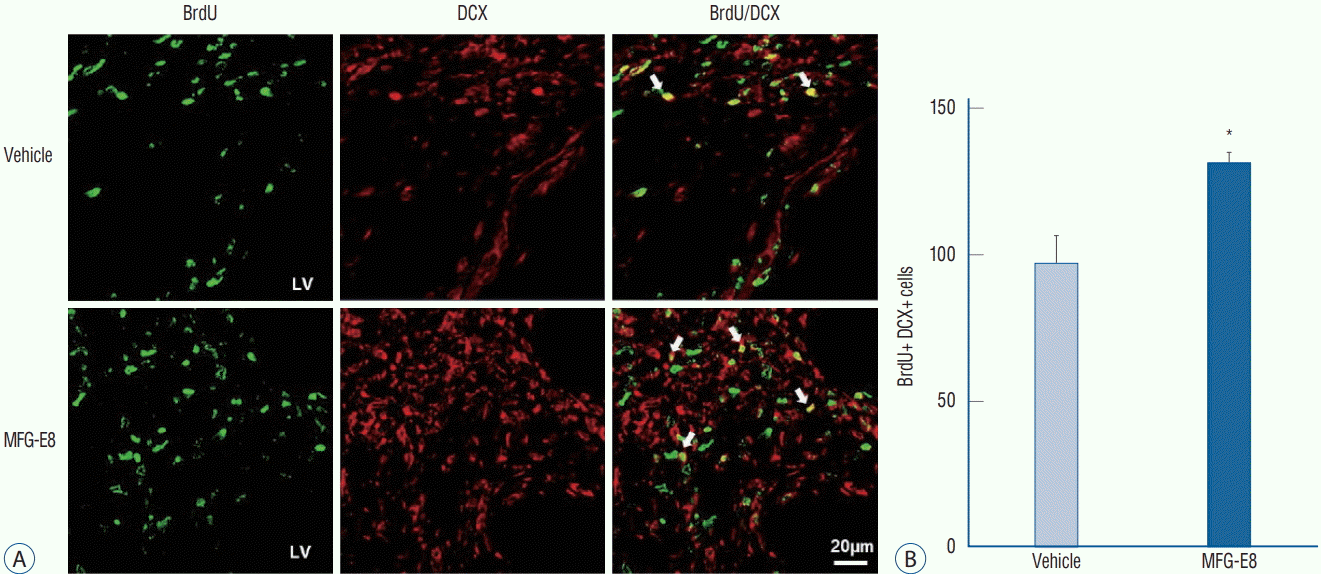 | Fig. 4.MFG-E8 promotes neurogenesis in ischemic rat brains. Effects of MFG-E8 treatment on neurogenesis were examined by immunostaining of BrdU (green) and DCX (red). A : BrdU/DCX double-positive cells in the SVZ on day 15 after stroke in the rat brain. Arrows indicate BrdU/DCX double-labeled cells. Scale bar=20 mm. B : The total number of BrdU/DCX double-positive cells in six sections of each animal was counted (n=4–5 per group). The number of BrdU/DCX double-positive cells was higher in the MFG-E8 treatment group than in the vehicle-treated group. Data are expressed as mean± SEM. *p<0.001, compared with the vehicle-treated group by Student’s t-test. BrdU : bromodeoxyuridine, DCX : doublecortin, MFG-E8 : milk fat globuleepidermal growth factor VIII, LV : lateral ventricle, SVZ : subventricular zone, SEM : standard error of mean. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download