INTRODUCTION
Compound comminuted depressed fracture (FCCD) of the kull is a type of depressed fracture with a laceration in the overlying scalp and galea in continuity with the fracture site and has been traditionally treated with surgery [
4,
5]. In a simple depressed skull fracture, there is no tear in the galea, and it is conventionally treated with surgical elevation only if the degree of depression is equal to or exceeds the thickness of the adjacent intact bone or if there is a degree of intracranial hematoma with mass effect that needs to be removed [
5]. Up to 6% of traumatic brain injuries (TBIs) may be complicated by depressed skull fractures, and FCCD accounts for up to 90% of these injuries [
4,
5,
19]. FCCD is associated with an infection rate of 1.9 to 10.6% [
5,
11,
19].
There is no doubt regarding the surgical treatment technique and timing of surgery for FCCD after TBI for a long time [
14]. To prevent infection in FCCD cases, it has been preferred to remove bone fragments, debride the wound, provide sufficient irrigation, and leave the cranial defect and wait for a sufficient period of time and then perform secondary cranioplasty [
5,
14]. The main reason for delayed cranioplasty was to reduce the risk of infection-related complications, such as sinusitis, osteomyelitis, meningitis, and empyema. However, recently, some studies have demonstrated that there is no difference in infection rate between immediate single-stage reconstruction of FCCD with titanium mesh and delayed secondary cranioplasty, and immediate single-stage reconstruction of FCCD with titanium mesh is a suitable surgical option with potential benefits in terms of cost-effectiveness, safety, and cosmetic and psychological outcomes. Moreover, the safety and feasibility of immediate titanium mesh implantation even in patients with postcraniotomy surgical site infection have been reported [
6,
18]. Here, the author performed immediate reconstruction with titanium mesh in consecutive patients with FCCD after TBI, reported the surgical results, and reviewed previous studies.
Go to :

MATERIALS AND METHODS
The study was approved by the Institute Ethical Committee of Wonkwang University Hospital (WKUH) and in compliance with institute's requirements (WKUH 201908013). The author retrospectively analyzed the medical records and radiological images of 19 consecutive patients who underwent single-stage reconstruction with titanium mesh for FCCD of the skull after TBI from April 2014 to June 2018. All patients with FCCD confirmed by computed tomography (CT) in the emergency room (ER) were included in the study. All surgeries were performed by one neurosurgeon.
The demographic characteristics of patients with FCCD, including sex, age distribution, cause of injury, time from damage to arrival in the hospital, time to surgery after arrival at the hospital, Glasgow coma scale (GCS) at admission and discharge, and hospital stay, were analyzed. The radiological demographic characteristics, including location and size of FCCD, FCCD associated lesions, and involvement of the paranasal sinus, were evaluated. FCCD-associated findings included skull fracture alone, acute epidural hematoma (AEDH), acute subdural hematoma (ASDH), traumatic subarachnoid hemorrhage (TSAH), and traumatic intracranial hemorrhage (TICH). The characteristics associated with surgery and infection, including the presence of dural tear, period of postoperative intravenous antibiotic administration, postoperative complications, changes in inflammatory markers and body temperature, and outcome, were investigated. Inflammatory markers included white blood cell (WBC; normal range, 4000–10000/µL), erythrocyte sedimentation rate (ESR; normal range, 0–20 mm/h), and C-reactive protein (CRP; normal range, 0–5 mg/L). All patients were followed over a period of 10 months after discharge to assess long-term outcome and complications.
Surgical technique
Prophylactic antibiotics with ceftriaxone (2 g per day) were injected intravenously in all patients preoperatively in the ER. All patients underwent sufficient irrigation with normal saline prior to surgical draping under general anesthesia. The author performed removal of foreign bodies and hematomas, debridement of damaged tissues, removal of fragmented bones, drilling of suspected contaminated bone, suture of the torn dura mater, meticulous hemostasis, and sufficient irrigations with normal saline mixed with antibiotics through lacerations in the overlying scalp. Three patients (patient No. 1, 4, and 15) underwent surgery through a bicoronal skin incision due to small sized skin wound and fractures of orbital wall. The author performed removal or cauterization of the mucosa of the opening of the frontal sinus and then obliteration of the rest of the frontal sinus using noncontaminated free fat, muscle, and pericranial flaps. Some of the bone fragments that could be used sufficiently because of their large size and mild depression were reconstructed using a titanium mini plate. The author performed primary reconstruction of the skull defect in all patients using titanium mesh plates, and it was fixed using several 4- or 5-mm titanium microscrews. Titanium microscrews were inserted through the mesh and into the underlying bone circumferentially to fix the mesh. The scalp and skin were closed with monofilament suture material. Immediately after the surgery, brain CT was performed to confirm that there was no additional postoperative hematoma. All patients were intravenously injected with ceftriaxone (2 g per day) for approximately 1–2 weeks postoperatively.
Go to :

DISCUSSION
The majority of previous studies supports the removal of contaminated fragmented bone and elevation of FCCD as soon as possible after TBI [
5,
11]. The rationale for immediate surgery of FCCD originates from its association with infection and late epilepsy [
5]. Jennett and Miller [
11] reported an incidence of infection of 10.6% in a series of 359 patients with compound cranial fractures. They revealed that these infections were associated with a remarkably higher incidence of persistent neurological deficit, late epilepsy, and death. They also demonstrated that proper removal and replacement of the fragmented bone were safe, and there was no difference in infection rate when fragmented bones were removed rapidly and adequately, even if the dura mater was torn. Immediate surgery in FCCD cases including surgical debridement reduced the infection rate to 4.6%. Therefore, they reported that this primary surgery may not be considered dangerous, and that avoiding delayed cranioplasty could be advantageous.
The traditional treatment in patients with FCCD includes wound debridement, elevation of the fracture, removal of bone fragments, evaluation of intracranial pathological findings, and delayed cranioplasty [
5,
14]. Most clinical concerns regarding single-stage reconstruction with cranioplasty were related to infection. Most of the studies supporting two-stage surgery with delayed cranioplasty were based on combat-related injuries that were significantly different from those of civilians [
9,
10,
17]. Combat-related injuries cause a higher degree of fragmentation and contamination than civilian trauma. However, numerous studies of civilian TBI have begun to counter the major concerns of infection in early primary single-stage surgery [
14]. Recently reported studies show that if surgery is performed rapidly, immediate replacement of the fragmented bone does not seem to increase the infection rate, and this primary replacement does not require subsequent cranioplasty and prevents its attendant risks and complications [
1,
3-
5,
14,
19]. Braakman [
4] reported that there were five cases (4.6%) of infection in 109 cases of replacement and the same number (8.9%) in 56 cases where the fragments had been completely removed in 225 consecutive patients with a depressed skull fracture. Wylen et al. [
19] also retrospectively reviewed 32 consecutive patients treated with debridement and elevation of compound depressed skull fractures with primary replacement of bone fragments within 72 hours of injury, and reported that there were no infectious sequelae; they concluded that immediate replacement of bone fragments in compound depressed skull fractures does not increase the risk of infectious complications. Blankenship et al. [
3] also reported that there were no instances of wound infection or osteomyelitis in a retrospective study of 31 consecutive children with compound depressed skull fractures treated with bone fragment replacement. Therefore, no child required subsequent cranioplasty. They proposed that bone fragment removal for compound depressed skull fractures, regardless of the degree of contamination, presence of dural laceration, or degree of intracerebral injury, is unnecessary and that bone fragment replacement avoids a second surgery for cranioplasty. Similarly, Adeloye and Shokunbi [
1] described a 0% infection rate in 12 patients with compound depressed skull fractures whose post-debridement cranial defects were treated with immediate bone replacement. They recommended primary repair of skull defects with bone fragments as the treatment of choice during debridement of compound depressed skull fractures of mild to moderate severity within 1 day of injury.
Additionally, surgeons have to consider cosmetic aspects and infection-related issues when deciding on the type of surgery. There are many alloplastic grafts available for cranioplasty. It has a variety of advantages and disadvantages depending on the materials. Polymethyl methacrylate is an acrylic resin that can be molded and provides strength and protection similar to that by the skull but has the disadvantage of an infection rate similar to that of cranioplasty with autogenous or autoclaved graft [
7]. Hydroxyapatite, a calcium-based bone cement, has the advantages of increased osteoconduction and osteointegration, but it can become infected and can cause severe foreign body inflammatory reaction and extrusion [
12]. In contrast, dynamic titanium mesh has good properties of high tensile strength and biological inactivity, and several studies have shown that the infection rate of cranioplasty with titanium mesh is low [
18]. Titanium spinal implants have been used for pyogenic and tuberculous spondylitis and discitis without causing persistent infection [
13]. Titanium is a widely used implant substance with excellent characteristics because of its adequate radiolucent, no magnetic, or paramagnetic properties and excellent biocompatibility [
14]. The improved complex mesh patterns can be more convenient and rapidly fit the implants to the contour of the skull properly for both small and large skull defects while maintaining strength [
14]. The frontal bone is one of the most important parts in terms of cosmetics aspect, even more so when including the fracture of orbital bone. In this study, there were five patients with fractures of orbital bone, all of whom were quickly and easily reconstruct using titanium mesh. These patients were also cosmetically satisfied and there were no postoperative complications (
Fig. 4).
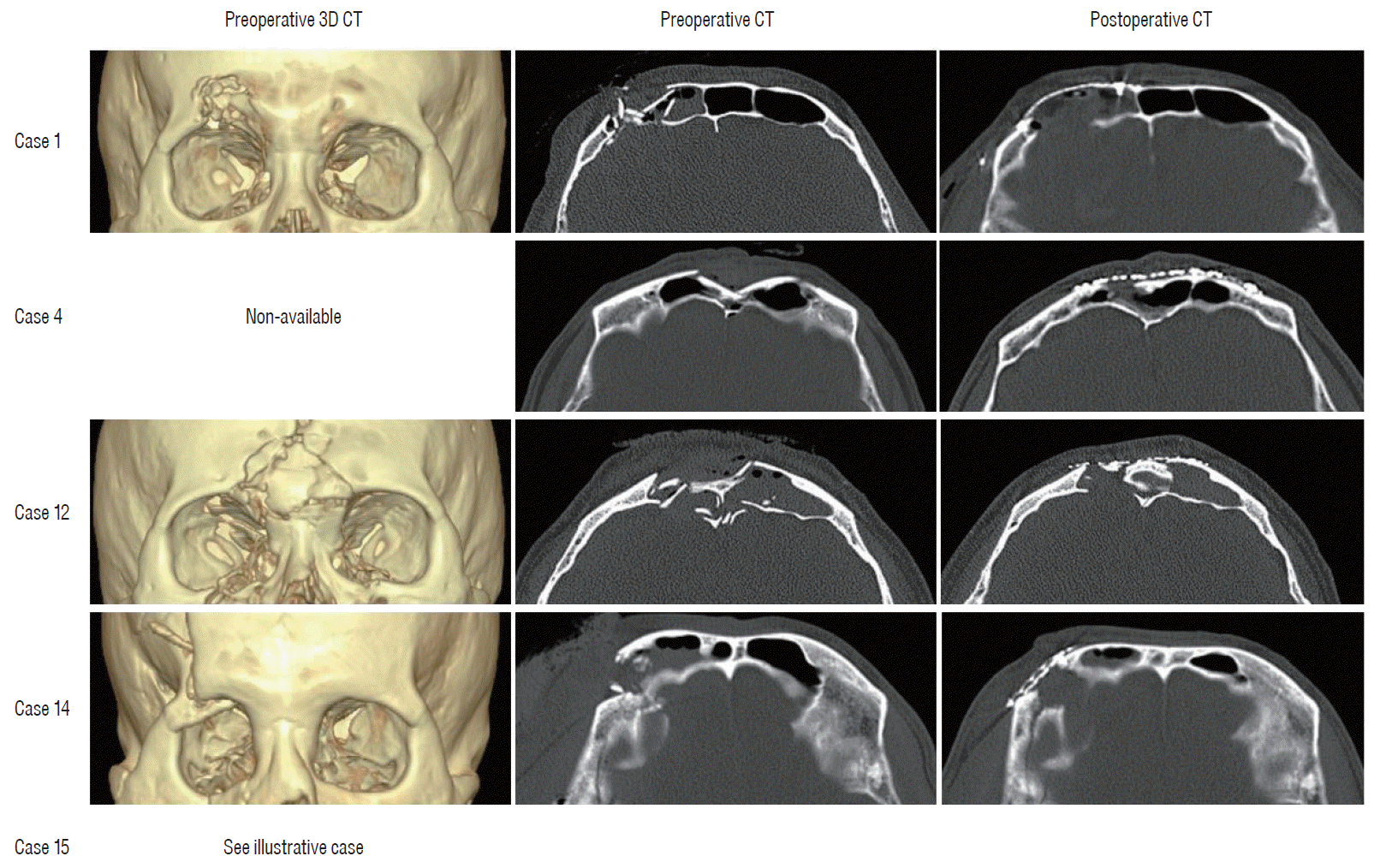 | Fig. 4.Preoperative and postoperative cranial CT of patients with fractures of orbital bone. CT : computed tomography. 
|
Marbacher et al. [
14] showed that primary reconstruction with titanium mesh for FCCD was feasible, safe, cost-effective, and cosmetically preferable than the conventional staged approach. This also prevents the risks and costs associated with a delayed cranioplasty, which is usually performed under general anesthesia [
18]. Titanium mesh implantation has been used for some postcraniotomy surgical infections. Ehrlich et al. [
6] and Wind et al. [
18] demonstrated that immediate titanium mesh cranioplasty is a cost-effective, safe, and cosmetically suitable alternative to delayed cranioplasty in selected patients and may be a suitable option for the treatment of postcraniotomy infections.
In this study, the mean duration from TBI to surgery was 7.0±3.9 hours, and all patients underwent surgery on the day of injury. This suggest that the immediate administration of antibiotics in the ER and rapid surgery after TBI reduced the likelihood of infectious complications. The mean hospital stay was 18 days, which was not significantly different compared to those in other types of traumatic neurosurgical diseases. However, since additional hospital stay for delayed secondary cranioplasty in two-stage surgery was not necessary, immediate single-stage reconstruction of FCCD with titanium mesh had potential advantages in terms of cost-effectiveness.
FCCD was frequently located in the frontal and parietal bones than in other regions. Of patients with FCCD in the frontal bone, 62.5% had paranasal sinus injury. Therefore, when performing surgery for FCCD in the frontal bone, it is necessary to pay close attention to the opening of the paranasal sinus. Several studies have been reported to determine whether antibiotics reduce the incidence of meningitis in patients with FCCD. Mendelow et al. [
15] reported that the early treatment with ampicillin and sulphonamide, in addition to adequate surgical debridement, is recommended in patient with compound depressed skull fractures in 176 patients with FCCD. Ali and Ghosh [
2] commented that the incidence of infectious complications other than meningitis in the non-antibiotic group was higher than in the group given antibiotics. There are some literatures that the benefit of prophylactic antibiotics in the FCCD could not be proved. Prakash et al. [
16] analyzed 453 patients with depressed skull fractures concluded that there was substantial data to support the use of prophylactic antibiotics in patients to reduce chances of infection with it. Haines examined that the evidence regarding antibiotic prophylaxis in five specific neurosurgical situations (clean surgical procedures, cerebrospinal fluid shunts, external venticulostomies, basilar skull fractures, and compound skull fractures) [
8]. In addition, he concluded that it was unclear whether all patients with FCCD required antibiotic treatment [
8]. Postoperatively, antibiotics were used for an average of 12.6 days. The mean WBC count remained within normal ranges pre- and postoperatively, and mean ESR and CRP count were slightly increased 7 days postoperatively and subsequently decreased. This temporary increase was not indicative of infection. There was no postoperative seizure or complications, such as infection or cerebrospinal fluid leakage, in all patients.
Go to :





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download