INTRODUCTION
MATERIALS AND METHODS
Patient selection
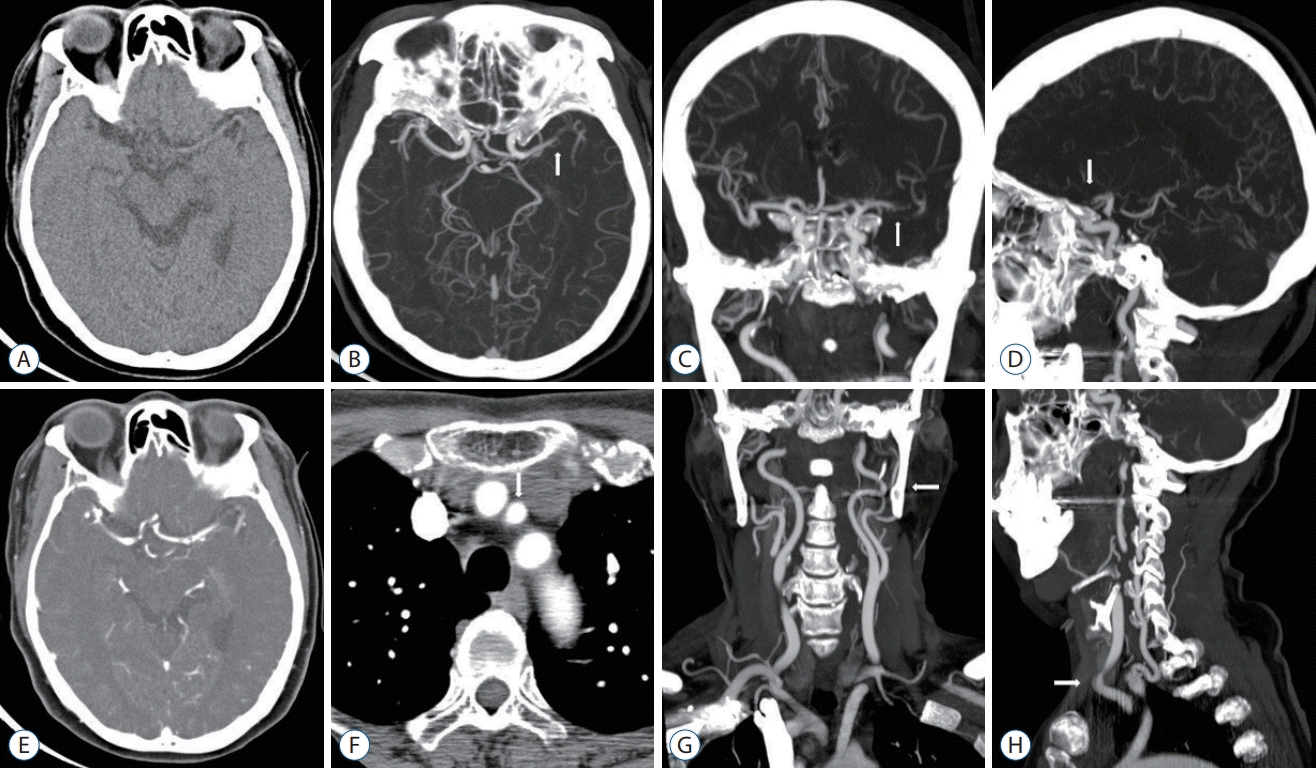 | Fig. 1.Single-phase CT angiography images including NCCT and MIP images. A 62-year-old woman with a left M1 MCA occlusion. A : Thin-section NCCT (no hypodense lesion, no hemorrage). B : MIP intracranial axial image (arrow, left M1 MCA occlusion). C : MIP intracranial coronal image (arrow, left M1 MCA occlusion). D : MIP intracranial sagittal image (arrow, left M1 MCA occlusion). E : Contrast-enhanced CT image. F : Axial image showing the aortic arch (arrow, left CCA; origin of Brachiocephalic trunk, left CCA, and left subclavian artery). G : MIP extracranial coronal image (arrow, no stenosis of left CCA-ICA and tortuosity of left cervical ICA). H : MIP extracranial sagittal image (arrow, tortuosity of left mid CCA). CT : computed tomography, NCCT : non-contrast computed tomography, MIP : maximum intensity projection, MCA : middle cerebral artery, CCA : common carotid artery, ICA : internal carotid artery. |
Image protocol and analysis
Single-phase CTA
Non-contrast CT and MRI
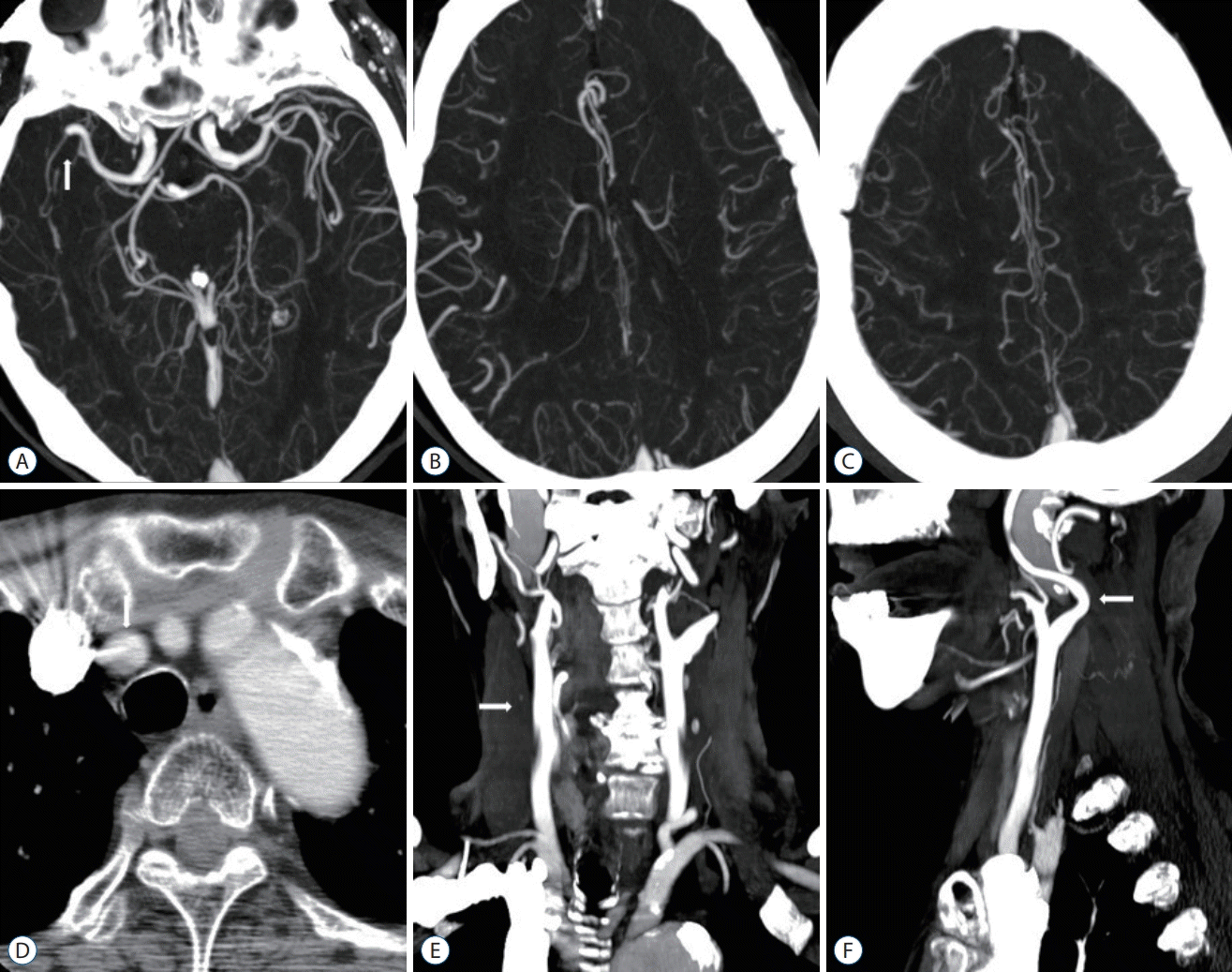 | Fig. 2.A 78-year-old woman with a right distal M1 MCA occlusion. OTD 240 minutes, NIHSS 16, DTI 16 minutes, DTP 82 minutes, mTICI 3, 90 days mRS 1. A : MIP intracranial axial image (arrow, right distal M1 MCA occlusion). B and C : Good pial collaterals (score 5). D : Axial image showing the aortic arch (arrow, Brachiocephalic trunk; origin of Brachiocephalic trunk, left CCA, and left subclavian artery). E : MIP extracranial coronal image (arrow, no significant stenosis of right CCA to cervical ICA). F : MIP extracranial sagittal image (arrow, tortuosity of proximal right cervical CCA). MCA : middle cerebral artery, OTD : onset-to-door time, NIHSS : National Institutes of Health Stroke Scale, DTI : door-to-image time, DTP : door-to-puncture time, mTICI : modified thrombolysis in cerebral infarction, mRS : modified Rankin Scale, MIP : maximum intensity projection, CCA : common carotid artery, ICA : internal carotid artery. |
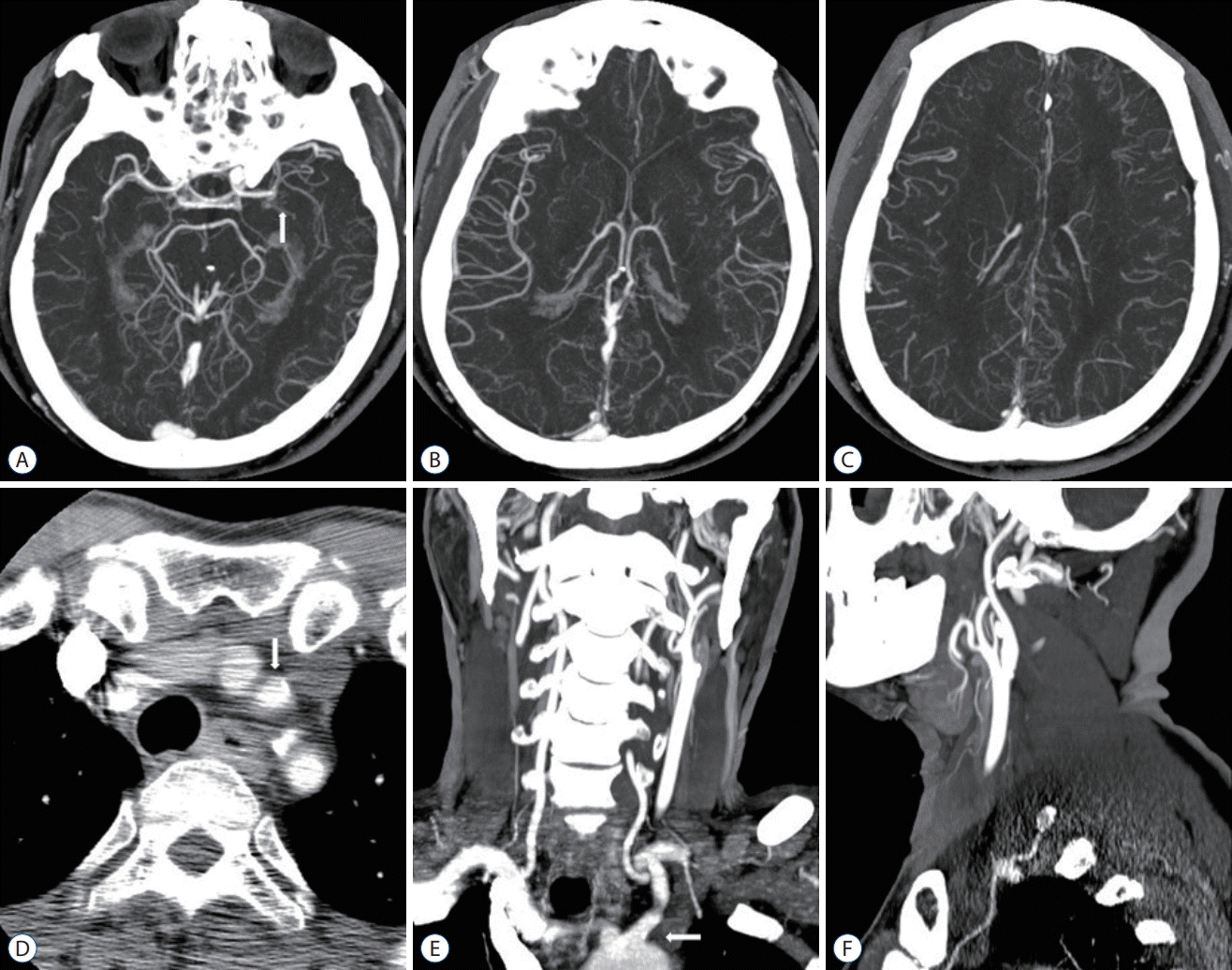 | Fig. 3.A 70-year-old man with a left mid M1 MCA occlusion. OTD 22 minutes, NIHSS 12, DTI 6 minutes, DTP 70 minutes, mTICI 2b, 90 days mRS 2. A : MIP intracranial axial image (arrow, left mid M1 MCA occlusion). B and C : Moderate pial collaterals (score 3). D : Axial image showing the aortic arch (arrow, left CCA; origin of Brachiocephalic trunk, left CCA, aberrant left VA, and left subclavian artery). E : MIP extracranial coronal image (arrow, bovine origin of left CCA). F : MIP extracranial sagittal image (no significant stenosis of left CCA to cervical ICA). MCA : middle cerebral artery, OTD : onset-to-door time, NIHSS : National Institutes of Health Stroke Scale, DTI : door-to-image time, DTP : door-to-puncture time, mTICI : modified thrombolysis in cerebral infarction, mRS : modified Rankin Scale, MIP : maximum intensity projection, CCA : common carotid artery, VA : vertebral artery, ICA : internal carotid artery. |
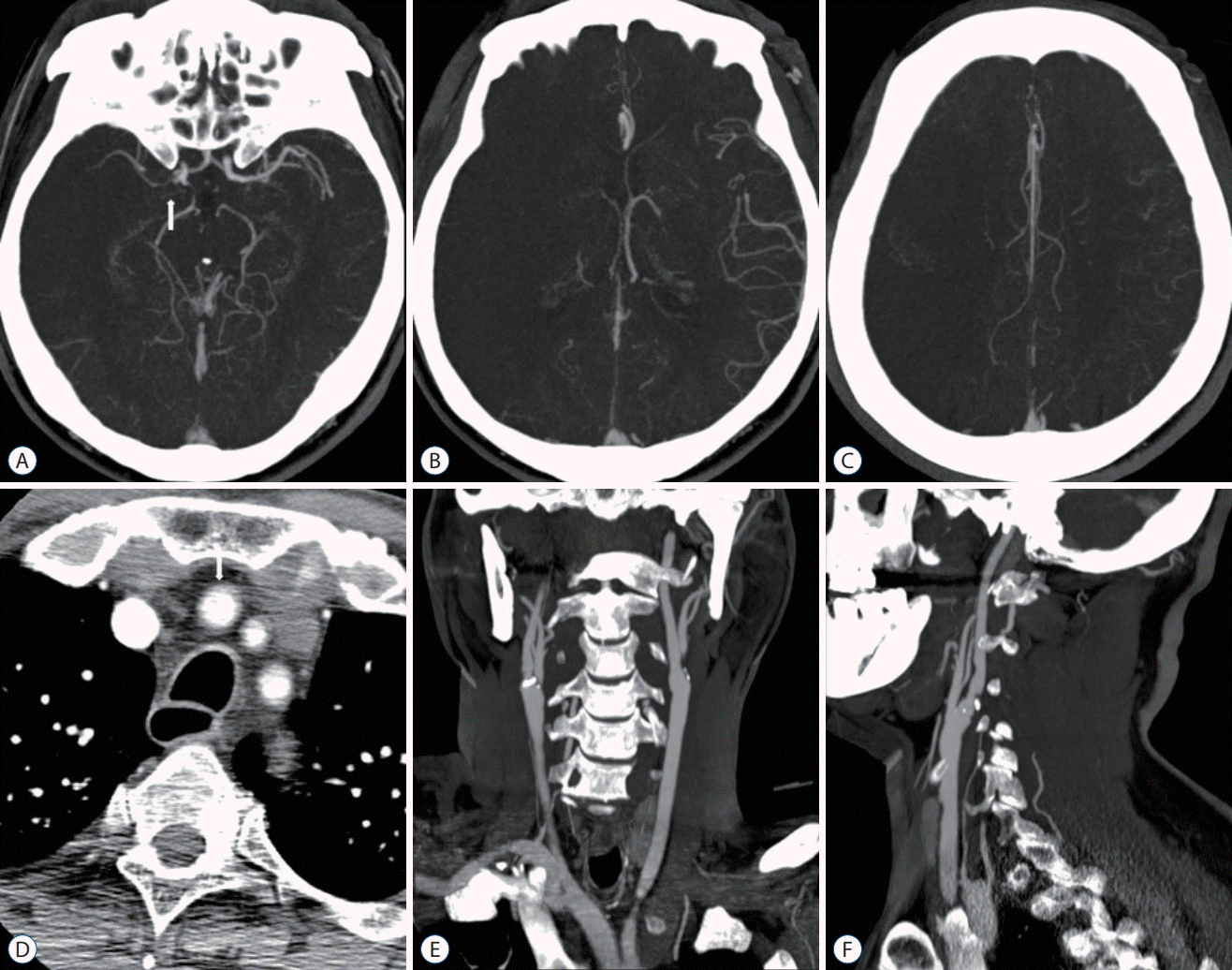 | Fig. 4.A 63-year-old man with a right proximal M1 MCA occlusion. OTD 48 minutes, NIHSS 18, DTI 14 mintues, DTP 67 minutes, mTICI 3, 90 days mRS 5. A : MIP intracranial axial image (arrow, right proximal M1 MCA occlusion). B and C : Poor pial collaterals (score 0). D : Axial image showing the aortic arch (arrow, Brachiocephalic trunk; origin of Brachiocephalic trunk, left CCA, and left subclavian artery). E and F : MIP extracranial coronal and sagittal images (no significant stenosis of right CCA to cervical ICA). MCA : middle cerebral artery, OTD : onset-to-door time, NIHSS : National Institutes of Health Stroke Scale, DTI : door-to-image time, DTP : door-to-puncture time, mTICI : modified thrombolysis in cerebral infarction, mRS : modified Rankin Scale, MIP : maximum intensity projection, CCA : common carotid artery, ICA : internal carotid artery. |
Table 1.
Endovascular procedure
Outcome measures
RESULTS
Table 2.
Values are presented as mean±standard deviation or number (%). r-tPA : recombinant tissue plasminogen activator, CTA : computed tomography angiography, NCCT : non-contrast computed tomography, MRi : magnetic resonance image, ICA : internal carotid artery, MCA : middle cerebral artery, ACA : anterior cerebral artery, PCA : posterior cerebral artery, BA : basilar artery, VA : vertebral artery
Table 3.
| CTA (n=34) | NCCT+MRi (n=33) | p-value | |
|---|---|---|---|
| Onset-to-door time | 109.0±119.1 | 141.0±137.2 | 0.308 |
| Door-to-image time | 13.0±6.8 | 93.0±30.8 | 0.000* |
| Door-to-puncture time | 86.0±22.1 | 177.0±47.5 | 0.000* |
| Door-to-reperfusion time | 122.0±32.7 | 211.0±50.1 | 0.000* |
| Onset-to-image time | 122.0±119.0 | 231.0±144.7 | 0.001* |
| Onset-to-puncture time | 195.0±128.0 | 314.0±157.6 | 0.001* |
| Onset-to-reperfusion time | 231.0±128.9 | 332.0±142.7 | 0.001* |
Table 4.
DISCUSSION
Table 5.
NIHSS : National Institutes of Health Stroke Scale, MT : mechanical thrombectomy, mTICI : modified thrombolysis in cerebral infarction, NCCT : noncontrast computed tomography, CT : computed tomography, CTA : computed tomography angiography, CTP : computed tomography perfusion, MRI : magnetic resonance image
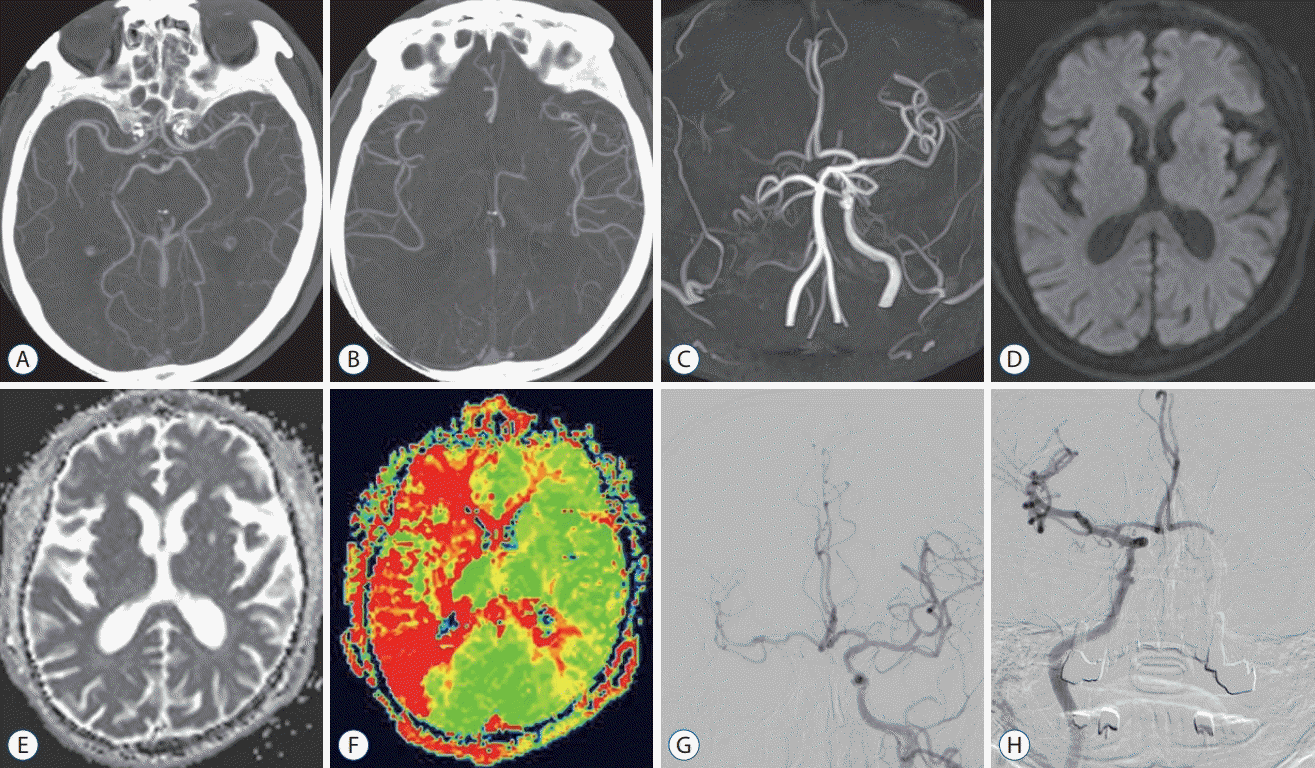 | Fig. 5.A 80-year-old man with a right proximal cervical ICA occlusion. OTD 304 minutes, NIHSS 21, DTI 78 minutes, DTP 164 minutes, mTICI 3, 90 days mRS 0. A and B : MIP intracranial axial image (no occlusion, good pial collaterals). C : MRA image (occlusion of ICA and weak flow of MCA). D-F : MRI images (perfusion-diffusion mismatch). G : Left ICA angiography (pre-MT, irrigation of contralateral ACA and MCA). H : Right ICA angiography (post-MT, successful recanalization, mTICI 3). ICA : internal carotid artery, OTD : onset-to-door time, NIHSS : National Institutes of Health Stroke Scale, DTI : door-toimage time, DTP : door-to-puncture time, mTICI : modified thrombolysis in cerebral infarction, mRS : modified Rankin Scale, MIP : maximum intensity projection, MRA : magnetic resonance angiography, MCA : middle cerebral artery, MRI : magnetic resonance image, MT : mechanical thrombectomy, ACA : anterior cerebral artery. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download