Abstract
Objective
The extensive vasa vasorum network functions as a conduit for the entry of inflammatory cells or factors that promote the progression of angiogenesis and plaque formation. Therefore, we investigated the correlation between the carotid vasa vasorum activities and carotid plaque vulnerability using indocyanine green video angiography (ICG-VA) during carotid endarterectomy (CEA).
Methods
Sixty-nine patients who underwent CEA were enrolled prospectively from September 2015 to December 2017. During CEA, a bolus of ICG was injected intravenously before and after resecting the atheroma. Additionally, we performed immunohistochemistry using CD68 (a surface marker of macrophages), CD117 (a surface marker of mast cells), and CD4 and CD8 (surface markers of T-cells) antibodies to analyze the resected plaque specimens.
Results
The density of active vasa vasorum was observed in all patients using ICG-VA. The vasa vasorum externa (VVE) and interna (VVI) were seen in 11 (16%) and 57 patients (82.6%), respectively. Macroscopically, the VVE-type patterns were strongly associated with preoperative angiographic instability (81.8%, p=0.005) and carotid plaque vulnerability (90.9%, p=0.017). In contrast, the VVI-type patterns were weakly associated with angiographic instability (31.6%) and plaque vulnerability (49.1%). CD68-stained macrophages and CD117-stained mast cells were observed more frequently in unstable plaques than in stable plaques (p<0.0001, p=0.002, respectively).
Conclusion
The early appearance of VVE, along with the presence of many microvessel channels that provided nutrients to the developing and expanding atheroma during ICG-VA, was strongly associated with unstable carotid plaques. The degree of infiltration of macrophages and mast cells is possibly related to the formation of unstable plaques.
Indocyanine green video angiography (ICG-VA) is commonly used during intracranial aneurysm surgery, bypass surgery, and carotid endarterectomy (CEA) to determine vascular patency and to obtain real-time lesional status. We recently published our experience of using ICG-VA during a variety of cerebrovascular surgeries, including CEA [26]. However, ICG-VA has been used mainly during CEA to identify the location and extent of carotid stenosis, which is caused by plaque in the carotid artery [9,17]. Extensive evaluation of the atherosclerotic plaque specimens collected during CEA consistently demonstrated that plaque composition is a major factor determining the risk of cerebral ischemia [6,8]. The transformation from a stable to an unstable atheroma is a consequence of complex molecular and cellular pathophysiological mechanisms. Vulnerable plaque is prone to rupture, because it has a large lipid necrotic core and a thin fibrous capsule [20,25].
Carotid atherosclerosis is a lipid-driven, systemic inf lammatory disease that affects large and medium-sized arteries. Atherosclerotic plaque develops because of the build-up of excess cholesterol, followed by its modification and subsequent plaque inf lammation. Cells of the innate and adaptive immune systems respond to the lipid components within the plaque; the recruited cells of corresponding systems subsequently infiltrate the plaque. Because of cell interactions and subsequent activation, atherosclerotic plaques progress towards rupture or severe stenosis [19,23,36].
In the present study, we focused on delineating the vasa vasorum of the carotid artery using ICG-VA. Furthermore, we investigated the relationships between preoperative radiological and pathological characteristics of carotid plaque.
In this study, the records of 69 patients with symptomatic carotid artery stenosis ≥50% or asymptomatic carotid artery stenosis ≥70% who consecutively underwent CEA between September 2015 and December 2017 and were prospectively examined at our institution were retrospectively reviewed. Surgery was indicated for the treatment of carotid stenosis adhering to the criteria of the North American Symptomatic Carotid Endarterectomy Trial [29] and the Asymptomatic Carotid Atherosclerosis Study [41]. All patients received detailed information about the following : 1) CEA, 2) carotid artery stenting, and 3) the best medical treatment and its specific advantages and potential complications.
Patients who had experienced a transient or permanent ipsilateral ocular or cerebral ischemic event within the past 6 months because of carotid artery stenosis were considered symptomatic. Exclusion criteria for the study were as follows : 1) an unstable neurological condition, 2) progressive stroke, 3) stenosis caused by dissection, or 4) a manifested infection such as pneumonia or a urinary tract infection.
Vasa vasorum were classified into three categories on the basis of previous studies [30,33]. Vasa vasorum externa (VVE) includes small arteries arising from major branches of adjacent arteries, and vasa vasorum interna (VVI) includes small arteries originating from the main lumen of arteries. Both VVE and VVI enter the vascular wall. Vasa vasorum venosum drain a network of capillaries/venules laid down around outer media to veins [30,33]. ICG-VA was used to identify these patterns.
Preoperative angiographic instability of a carotid stenosis was defined by the presence of luminal irregularities (i.e., ulcerations and gravel road shape), and it was reviewed by two experienced neurosurgeons who were not involved in surgery and were blinded to the patient data.
Plaque vulnerability was defined on the basis of the following intraoperative plaque characteristics : 1) a large lipid core, 2) thin fibrous cap, 3) inflammatory changes at the shoulder of the fibrous cap, and 4) decreased number of smooth muscle cells within the fibrous cap [18,32].
This study design was approved by the Institutional Review Board of Chonnam National University Hospital (CNUH-2014-010), and all the participants provided informed consent.
ICG-VA was performed to visualize the cervical carotid artery stenosis, and the contrast filling patterns were analyzed. Intraoperative ICG-VA was performed us ing an OPMI®PenteroTM surgical microscope (Carl Zeiss, Jena, Germany). The recommended dose of ICG dye for VA is 0.2–0.5 mg/kg, and the maximum daily dose is 5 mg/kg. The unit was positioned at a distance of 30–40 cm from the area of interest to carry out the measurements. Care was taken to ensure uniform illumination of the area of interest by the laser light source. When requested by a surgeon, ICG was administered intravenously at the time of angiography by an an esthesiologist, before carotid arteriotomy. A half-dose bolus (0.2–0.3 mg/kg) was used (a standard dose of 25 mg dis solved in 5 mL water). Two patients who underwent ICG-VA of cervical vessels during high-flow bypass of a ruptured cerebral aneurysm acted as controls.
The carotid plaques were analyzed by immunohistochemistry. Paraformaldehyde-fixed sections of the specimens were embedded in paraffin. Serial sections were prepared on silanecoated glass slides, and they were immunostained with CD68 (1:20, mouse monoclonal, Clone JC70 A; DAKO, Glostrup, Denmark), CD117 (1:20, mouse monoclonal, Clone KP1; DAKO), CD4, and CD8 antibodies using a BOND-MAX autostainer (Leica Microsystems, Buffalo Grove, IL, USA). Images were acquired using a Keyence BZ-9000 microscope (Keyence Co., Osaka, Japan). Image J software (National Institutes of Health, Bethesda, MD, USA) was used for the semiquantitative analysis of the percentages of CD68-, CD117-, CD4-, and CD8-stained cells on the basis of the stained area. Histopathological analysis was performed by an experienced pathologist who was blinded to the patient characteristics and imaging findings.
All data are expressed as mean±standard deviation. Nominal variables are expressed as numbers and percentages. The statistical analysis for the stained positive areas was performed with analysis of variance, followed by the Newman-Keuls post-hoc test or Dunnett’s test using GraphPad Prism software (GraphPad Inc., San Diego, CA, USA). Categorical data were compared using two-tailed chi-square statistic with the Yates’s correction or Fisher’s exact test. The statistical significance threshold was set at p<0.05.
We retrospectively reviewed the records of 69 patients who consecutively underwent elective CEA with intraoperative ICG-VA between September 2015 and December 2017 from our prospectively archived database. The patient population consisted of 51 male (79.7%) and 14 female (20.3%) participants. The patients’ age ranged from 47 years to 82 years (mean age, 68.5±2.5 years). Clinical baseline characteristics of the patient are detailed in Table 1.
In control patients undergoing ICG-VA, the vascular lumen was first filled with ICG; consequently, the arterial component of the vasa vasorum slowly appeared, followed by the appearance of the venous component; finally, uniform filling of the arterial wall observed. However, no dominant flow enhancement in the carotid artery was detected during observations (Supplementary Fig. 1, Supplementary Video 1).
The density of active vasa vasorum was observed in all the cases (n=69) during the ICA-VA series. Three different vasa vasorum patterns were observed, which are schematically represented in Fig. 1. We categorized the three depiction patterns as follows : 1) VVE, the vasa vasorum of the carotid adventitia was depicted first, followed by augmentation of ICG in the wall and partially in the inner wall (n=11, 16.0%; Fig. 2, Supplementary Video 2); 2) VVI, augmentation of ICG in the wall and inner wall was depicted prior to or simultaneously with the depiction of vasa vasorum of the carotid adventitia (n=57, 82.6%; Fig. 3, Supplementary Video 3); and 3) vasa vasorum venosum (VVV), early appearance of ICG in the carotid lumen and inner wall was depicted prior to or simultaneously with the depiction of vasa vasorum of the carotid adventitia (n=1, 1.4%; Fig. 4, Supplementary Video 4).
We analyzed the correlations of the different vasa vasorum descriptions with preoperative angiographic findings and postoperative plaque characteristics. The VVE-type patterns were strongly associated with preoperative angiographic instability (81.8%, p=0.005) and carotid plaque vulnerability (90.9%, p=0.017) during the operation. In contrast, the VVI-type patterns were weakly associated with angiographic instability (31.6%) and plaque vulnerability (49.1%). Nonetheless, preoperative angiographic instability was consistent with intraoperative plaque vulnerability (Supplementary Table 1, percent agreement=80.6%, κ score=0.62).
Resected plaque stained with CD68 was significantly more frequently observed in the unstable plaque (p<0.0001); additionally, the CD117-positive areas were significantly more prominent in unstable plaque (p=0.0002; Supplementary Figs. 2 and 3). However, no significant differences were observed in the frequencies of CD4 or CD8 T-cells between stable and unstable plaques (p=0.18 and p=0.20, respectively; Fig. 5).
The aim of our study was to investigate the relationship between vasa vasorum activity and carotid plaque vulnerability during CEA. We found that ICG-VA presented different patterns on the basis of the depiction of the carotid wall and the cases reviewed. The early depiction of adventitial vasa vasorum was consistently associated with highly unstable plaque, which was likely to hemorrhage or produce a migrating embolus intraoperatively [38,39].
Three types of vasa vasorum were proposed in 1960 on the basis of bovine aortic studies [33]. Microvessels arising from the vasa vasorum, under normal conditions, are limited to the adventitia and outer media, and they do not go beyond the intima [4].
Several modalities, such as contrast-enhanced ultrasound [1,5,35], magnetic resonance imaging [14], and fluorescein sodium VA, are useful for imaging the vasa vasorum [13]. A few studies have reported the status of the vasa vasorum using ICG-VA during CEA [12,13]. Many surgeons have recommended the use of ICG-VA to make intraoperative assessments, because this technique helps acquire real-time high-resolution images that depict blood flow in large and small vessels [3,16,17,26,31]. Intraoperative ICG-VA is a simple noninvasive technique that is used to identify surrogate markers of vulnerable carotid plaques. The accuracy of intraoperative ICG-VA in visualizing newly formed vasa vasorum has been confirmed by histological studies on CEA specimens [13]. We recently published our experience of using ICG-VA during a variety of cerebrovascular surgeries [26], and we have extended our understandings of CEA.
We continuously observed the delineation of the external appearance of the carotid wall under a microscope during the ICG-VA of the patients while they were undergoing CEA. In general, the vasa vasorum was filled with ICG in a virtually radiating conformation along with the long axis of the carotid artery, which is mainly around the area of carotid bifurcation. After the intraoperative filling of the carotid lumen, the arterial component of the vasa vasorum was quickly filled with ICG, because a non-homogenous vascular network was formed by the branches of the vasa vasorum in the area corresponding to the atheromatous plaque. Subsequently, the venous component was filled, and finally, the entire carotid wall was filled with ICG in a patchy fashion [12]. Generally, the carotid lumen was filled with ICG, followed by the delayed phase of filling the carotid wall vasa vasorum. The time interval between the depiction of the carotid wall/lumen and that of the delayed phase of the vasa vasorum was slightly longer when there was an unstable plaque than when there was a stable plaque. ICG-VA uniquely permits direct, real-time visualization of the neovasculature of carotid atherosclerotic plaque and associated adventitial vasa vasorum. Thus, we hypothesized that the normal activity of the carotid artery vasa vasorum had transformed and that the transformed activity facilitates nutrient supply during the development of carotid atherosclerosis.
Schoenenberger and Mueller first described three types of vasa vasorum, that is, the VVE, VVI, and VVV, which—in an animal experiment—resembled a tree-like structure in the arterial wall [33]. According to them, the VVE arises from the major branches of adjacent arteries, and the VVI from the main lumen of arteries. The adventitial, medial, and intimal atherosclerotic plaques of the carotid artery have their own nutritional supply such as the vasa vasorum, which is a network of small microvessels. The vasa vasorum increases when atherosclerosis develops, and it includes plaque neovascularization, which plays an important role in the progression of atherosclerosis. The extensive vasa vasorum network functions as a conduit for the entry of inflammatory cells or the factors that promote the progression of angiogenesis and plaque formation [28]. Fleiner et al. [7] reported a close relationship between a dense vasa vasorum vascular network and a strong inflammatory reaction within the vascular wall and symptomatic atherosclerosis. Furthermore, Moreno et al. [27] reported that the vessel wall and plaque microvessels increase in ruptured atherosclerotic plaques, suggesting a link between microvessels and plaque instability. Therefore, we hypothesized that a plaque with an early and dense depiction of adventitial vasa vasorum by ICG-VA (e.g., VVE) would be vulnerable and fragile, because the microvascular network plays an important role as a nutritional conduit. It is well known that intraplaque hemorrhage is derived mainly from adventitial vasa vasorum in the coronary arteries [15,40]. Furthermore, in the current study, the VVE was depicted first and the carotid wall/lumen was depicted second in the most unstable carotid plaque. Neovascularization of plaque and formation of the VVE are the most important predictors of carotid plaque vulnerability. Development of the adventitial vasa vasorum, which finally envelopes the atheroma, facilitates additional nutritional supply for plaque growth in the later stages. Feinstein was the first researcher to identify the adventitial vasa vasorum and neovascularization in carotid atherosclerotic plaque in vivo [5].
Interestingly, we found that plaques associated with a delayed depiction of the adventitial vasa vasorum by ICG-VA (e.g., VVI) showed a higher number of and denser microvessels than the plaques associated with an early depiction of the adventitial vasa vasorum (e.g., VVE), suggesting that they may also be vulnerable and fragile. Contrary to our expectation, no correlation was observed between the density of microvessels and vulnerability of plaque (31/57, 54.39%). However, a significant correlation was detected between the density of microvascularity and >70% carotid stenosis (56/57 98.2%). This result may have been a consequence of the small sample size; thus, further studies are needed.
Several studies on resected carotid atheroma have reported the presence of intraplaque neovessels and subsequent hemorrhage in the symptomatic period from the long-lasting asymptomatic stages of carotid artery stenosis [11,24]. During the past decade, most clinicians have considered atherosclerosis as a bland lesion. However, we have come to appreciate a prominent role played by inflammation in atherosclerosis and associated complications [21]. Although luminal narrowing due to atheroma bulk was focused on previously, the current understanding highlights the biological attributes of atheroma formation as inflammatory and immune responses.
Macrophages are innate immune effectors and are abundant in ruptured atherosclerotic plaques. Moreover, an atherosclerotic plaque contains a characteristic inflammatory infiltrate that includes monocytes, numerous monocyte-derived macrophages, modified lipid-laden macrophages (foam cells), and T-lymphocytes [10,22]. Thus, macrophages are critical to atherosclerotic inflammation [21,22].
With regard to immune cells in atherosclerotic lesions, although macrophages are the most abundant cells, T-lymphocytes constitute up to 20% of the cells [23]. Plaque T-lymphocytes have an interesting immunophenotypic pattern. Many plaque T-lymphocyte are a mixture of CD4-positive helper T cells and CD8-positive cytotoxic T cells [10]. Interestingly, neutrophils and B-lymphocytes are rarely found in atherosclerotic plaques; however, they are usually found during chronic inf lammation [10]. The pivotal role of vascular inf lammation in the progression of carotid plaques has been well documented. Vulnerable atherosclerotic plaques increase inflammation [21,22]. They usually have a large and soft lipid core (necrotic core) with a thin fibrous cap, and they have more macrophages than unruptured atherosclerotic plaques do [2,22].
Many investigators have considered the detection of neovascularization as a histological validation of plaque inflammation. The frequency of occurrence of microvessels stained with CD31 (endothelial marker) is observed to be significantly higher in unstable neovascularized plaque [13,34]. CD68 (macrophage marker), CD34 (endothelial marker), and CD66 (neutrophils) inf lammatory cells are also, reportedly, associated with intraplaque neovascularization [37].
In summary, the presence of these cells indicates that the innate and adaptive immune responses may be involved in plaque formation and progression, as well as plaque vulnerability.
This study has limitations that should be addressed. First, the main limitation of this study is the small sample size. Further studies with larger sample sizes are warranted to elucidate the reliability of correlation between ICG-VA findings and plaque vulnerability. Second, the analysis of the vasa vasorum pattern based on ICG-VA may not be quantitative. Therefore, the atheroma thickness and calcification may have affected the interpretation. Third, we classified all plaques, as stable and unstable plaques, angiographically and pathologically. It would be interesting to evaluate if there was a difference between the vasa vasorum patterns in symptomatic and asymptomatic patients.
The early appearance of the VVE, along with the presence of numerous microvessel channels that provide nutrients to the developing and expanding intima, during ICG-VA was strongly associated with the carotid plaque being unstable; these ICG-VA characteristics potentially indicate an unstable hemorrhagic environment prone to rupture. Macrophages and mast cells are involved in the formation of microvessels in an atherogenic plaque, and they accelerate plaque progression, leading to the formation of an unstable plaque. T lymphocytes are unlikely to be involved in this process. A further understanding of how these inf lammatory cells are regulated is needed to design specific immunomodulatory therapies for carotid atherosclerosis.
Notes
ACKNOWLEDGMENTS
We thank all the patients of the department of Neurosurgery who have donated the study samples. We also thank the researchers Dong-Jun Song and Da-Ae Kim for their invaluable support in patient management throughout the study.
This study was supported by a grant (CRI 14009-1) from Chonnam National University Hospital Biomedical Research Institute.
Supplementary Materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2019.0077.
Supplementary Fig. 1.
Indocyanine green video angiography (ICG-VA) findings of a control patient. No vasa vasorum activity was seen on the carotid artery.
Supplementary Fig. 2.
Representative case of carotid atheroma with CD68-stained macrophages. A : Carotid atherosclerosis developed in the intimal area during the early stage (cadaver study). B : Stable plaque showing a scantily stained area. C : Unstable plaque showing a much more abundantly stained area. Arrowheads indicate CD68-positive area (brown). A : adventitia, M : media, I : intima.
Supplementary Fig. 3.
Representative case of carotid atheroma with CD117-stained mast cells. A : Carotid atherosclerosis developed in the intimal area during the early stage (cadaver study). B : Stable plaque showing very scantily stained area. C : Unstable plaque showing an area that is much more abundantly stained than the stained area of a stable plaque but less stained than the area stained with CD68 (brown). Arrowheads indicate CD117-positive area. A : adventitia, M : media, I : intima.
Supplementary Table 1.
Angiographic, intraoperative findings of plaque and their relationship
Supplementary Video 1.
Uniform indocyanine green filling of the arterial wall. No vasa vasorum activity was seen on the carotid artery.
Supplementary Video 2.
00:00–00:05 : Early depiction of the vasa vasorum externa. 00:06–End : Augmentation of indocyanine green in the wall and partially in the inner wall.
References
1. Alonso A, Artemis D, Hennerici MG. Molecular imaging of carotid plaque vulnerability. Cerebrovasc Dis. 39:5–12. 2015.

2. Boyle JJ. Association of coronary plaque rupture and atherosclerotic inflammation. J Pathol. 181:93–99. 1997.

3. Dashti R, Laakso A, Niemelä M, Porras M, Hernesniemi J. Microscopeintegrated near-infrared indocyanine green videoangiography during surgery of intracranial aneurysms: the Helsinki experience. Surg Neurol. 71:543–550. discussion 550. 2009.

4. Doyle B, Caplice N. Plaque neovascularization and antiangiogenic therapy for atherosclerosis. J Am Coll Cardiol. 49:2073–2080. 2007.

5. Feinstein SB. Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J Am Coll Cardiol. 48:236–243. 2006.

6. Fisher M, Paganini-Hill A, Martin A, Cosgrove M, Toole JF, Barnett HJ, et al. Carotid plaque pathology: thrombosis, ulceration, and stroke pathogenesis. Stroke. 36:253–257. 2005.

7. Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 110:2843–2850. 2004.

8. Gronholdt ML. Ultrasound and lipoproteins as predictors of lipid-rich, rupture-prone plaques in the carotid artery. Arterioscler Thromb Vasc Biol. 19:2–13. 1999.

9. Haga S, Nagata S, Uka A, Akagi Y, Hamada Y, Shono T. Near-infrared indocyanine green videoangiography for assessment of carotid endarterectomy. Acta Neurochir (Wien). 153:1641–1644. discussion 1644. 2011.

10. Hansson GK, Libby P, Schönbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 91:281–291. 2002.

11. Hiyama T, Tanaka T, Endo S, Komine K, Kudo T, Kobayashi H, et al. Angiogenesis in atherosclerotic plaque obtained from carotid endarterectomy: association between symptomatology and plaque morphology. Neurol Med Chir (Tokyo). 50:1056–1061. 2010.

12. Kamiyama K, Nakagawara J, Takada H, Fumoto K, Osato T, Nakamura H. Transformation of the vasa vasorum from normal vessel structures to atheroma nutrient vessels on ICG angiography of cervical carotid artery stenosis. Surg Cereb Stroke (Jpn). 39:413–419. 2011.

13. Katano H, Yamada K, Sakurai K, Takahashi S. Depiction of the vasa vasorum during carotid endarterectomy by intraoperative videoangiography. J Stroke Cerebrovasc Dis. 23:2920–2927. 2014.

14. Kawahara I, Morikawa M, Honda M, Kitagawa N, Tsutsumi K, Nagata I, et al. High-resolution magnetic resonance imaging using gadolinium-based contrast agent for atherosclerotic carotid plaque. Surg Neurol. 68:60–65. discussion 65-66. 2007.

15. Kumamoto M, Nakashima Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol. 26:450–456. 1995.

16. Lane B, Bohnstedt BN, Cohen-Gadol AA. A prospective comparative study of microscope-integrated intraoperative fluorescein and indocyanine videoangiography for clip ligation of complex cerebral aneurysms. J Neurosurg. 122:618–626. 2015.

17. Lee CH, Jung YS, Yang HJ, Son YJ, Lee SH. An innovative method for detecting surgical errors using indocyanine green angiography during carotid endarterectomy: a preliminary investigation. Acta Neurochir (Wien). 154:67–73. discussion 73. 2012.

19. Legein B, Temmerman L, Biessen EA, Lutgens E. Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci. 70:3847–3869. 2013.

20. Li ZY, Howarth SP, Tang T, Gillard JH. How critical is fibrous cap thickness to carotid plaque stability? A flow-plaque interaction model. Stroke. 37:1195–1199. 2006.

23. Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 38:1092–1104. 2013.

24. Mofidi R, Crotty TB, McCarthy P, Sheehan SJ, Mehigan D, Keaveny TV. Association between plaque instability, angiogenesis and symptomatic carotid occlusive disease. Br J Surg. 88:945–950. 2001.

25. Mono ML, Karameshev A, Slotboom J, Remonda L, Galimanis A, Jung S, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis. 34:343–350. 2012.

26. Moon HS, Joo SP, Seo BR, Jang JW, Kim JH, Kim TS. Value of indocyanine green videoangiography in deciding the completeness of cerebrovascular surgery. J Korean Neurosurg Soc. 53:349–355. 2013.

27. Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 110:2032–2038. 2004.

28. Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 100:4736–4741. 2003.

29. North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 325:445–453. 1991.

31. Roessler K, Krawagna M, Dörfler A, Buchfelder M, Ganslandt O. Essentials in intraoperative indocyanine green videoangiography assessment for intracranial aneurysm surgery: conclusions from 295 consecutively clipped aneurysms and review of the literature. Neurosurg Focus. 36:E7. 2014.

32. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 340:115–126. 1999.
33. Schoenenberger F, Mueller A. On the vascularization of the bovine aortic wall. Helv Physiol Pharmacol Acta. 18:136–150. 1960.
34. Shah F, Balan P, Weinberg M, Reddy V, Neems R, Feinstein M, et al. Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc Med. 12:291–297. 2007.

35. Staub D, Schinkel AF, Coll B, Coli S, van der Steen AF, Reed JD, et al. Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging. 3:761–771. 2010.
36. Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 17:1410–1422. 2011.

37. Willems S, Vink A, Bot I, Quax PH, de Borst GJ, de Vries JP, et al. Mast cells in human carotid atherosclerotic plaques are associated with intraplaque microvessel density and the occurrence of future cardiovascular events. Eur Heart J. 34:3699–3706. 2013.

38. Yamada K, Yoshimura S, Kawasaki M, Enomoto Y, Asano T, Hara A, et al. Embolic complications after carotid artery stenting or carotid endarterectomy are associated with tissue characteristics of carotid plaques evaluated by magnetic resonance imaging. Atherosclerosis. 215:399–404. 2011.

39. Yoshida K, Narumi O, Chin M, Inoue K, Tabuchi T, Oda K, et al. Characterization of carotid atherosclerosis and detection of soft plaque with use of black-blood MR imaging. AJNR Am J Neuroradiol. 29:868–874. 2008.

40. Zhang Y, Cliff WJ, Schoefl GI, Higgins G. Immunohistochemical study of intimal microvessels in coronary atherosclerosis. Am J Pathol. 143:164–172. 1993.
41. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 273:1421–1428. 1995.
Fig. 1.
Illustration showing the three vasa vasorum patterns based on surface anatomy or indocyanine green-video angiography (ICG-VA) appearance. Modified according to Schoenenberger and Mueller [33]. I : intima, M : media, A : adventia.

Fig. 2.
Representative case of vasa vasorum externa. A 67-year-old woman presented with repeated episodes of left-sided motor weakness and dysarthria. A : Diffusion-weighted magnetic resonance image showing an acute infarction on the insular cortex. B : Carotid artery angiogram showing the unstable plaque (arrows). C : Indocyanine green video angiography image demonstrating an early depiction of the vasa vasorum externa (arrows). D : Intraoperative findings indicating unstable plaque—a necrotic core within the atheroma.
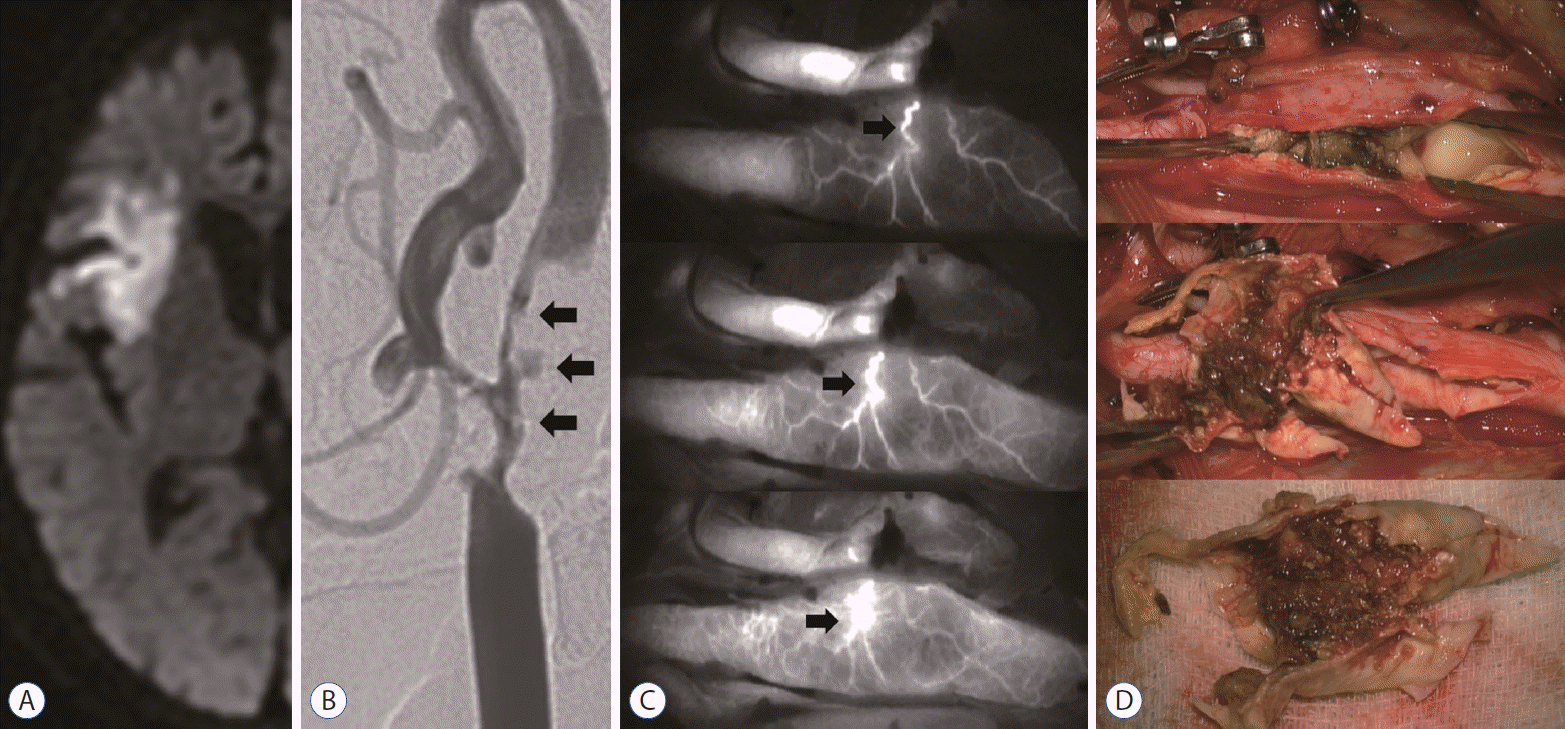
Fig. 3.
Representative case of vasa vasorum interna (VVI). A 65-year-old man presented with dysarthria and left hemiparesis grade II/IV and NIHSS 3. A : Diffusion-weighted magnetic resonance image showing a multifocal acute infarction on the right hemisphere. B : Carotid artery angiogram showing a carotid stenosis with a stable plaque (arrowheads). C : ICG-VA image demonstrating a delayed depiction of the VVI with abundant vascular channels (arrowheads). Postoperative ICG-VA image showing disappearance of the vasa vasorum vascular networks (bottom). D: Intraoperative findings indicating stable plaque—a smooth luminal surface (circle). NIHSS : National Institutes of Health Stroke Scale, ICG-VA : indocyanine green video angiography.
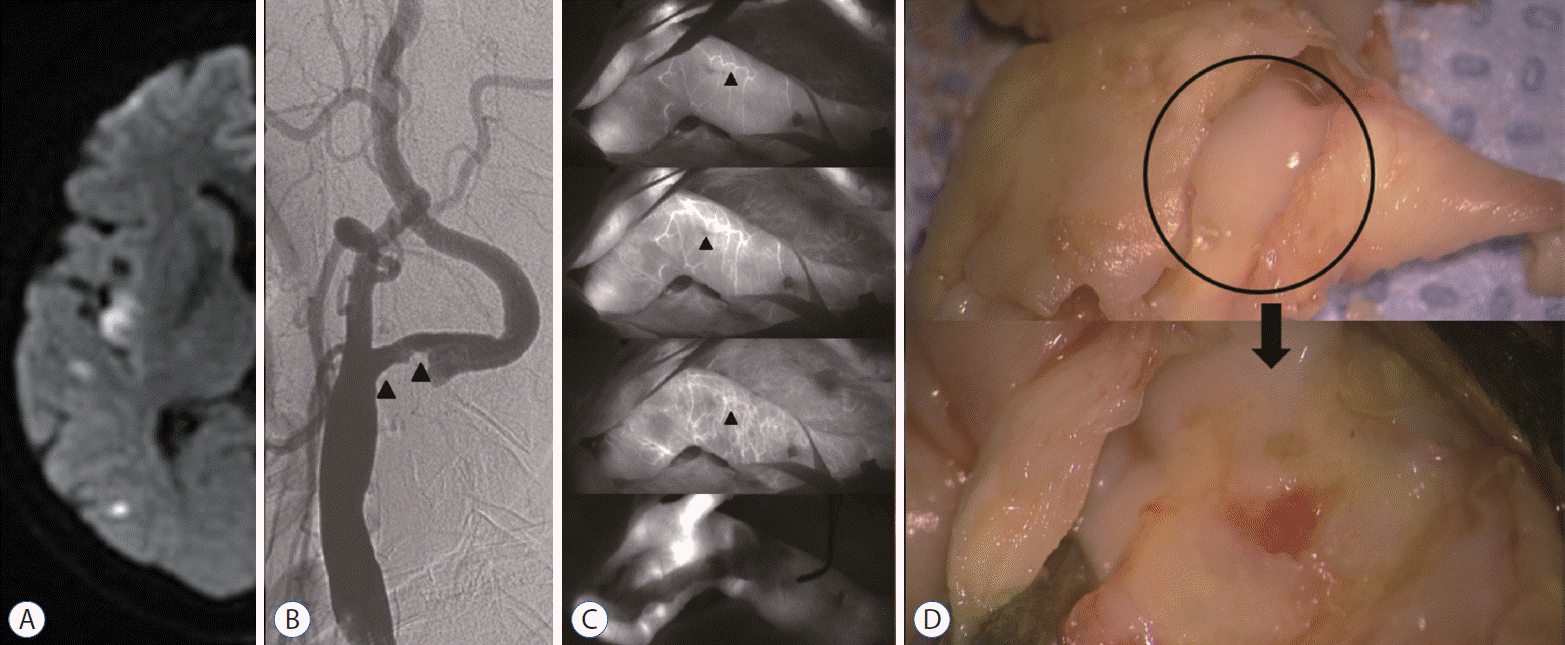
Fig. 4.
Representative case of vasa vasorum venosum (VVV). A 64-year-man presented with a recurrent a transient ischemic attack on the left side with a history of smoking. A : Carotid artery angiogram showing carotid stenosis (60%, the North American Symptomatic Carotid Endarterectomy Trial). B : Indocyanine green-video angiography image demonstrating delayed depiction of the VVV (arrowheads) along with the carotid bulb. C : Intraoperative findings indicating stable plaque—smooth outer and inner surfaces.

Fig. 5.
Depiction of inflammatory cell (macrophages and mast cells) activities in a resected plaque by immunohistochemistry studies. CD68-stained macrophages and CD117-stained mast cells were more frequently observed in unstable plaque, than in stable plaque (p<0.0002, p<0.0001, respectively). However, no significant differences in the frequencies of occurrence of CD4 or CD8 T-cells were observed between stable and unstable plaques.
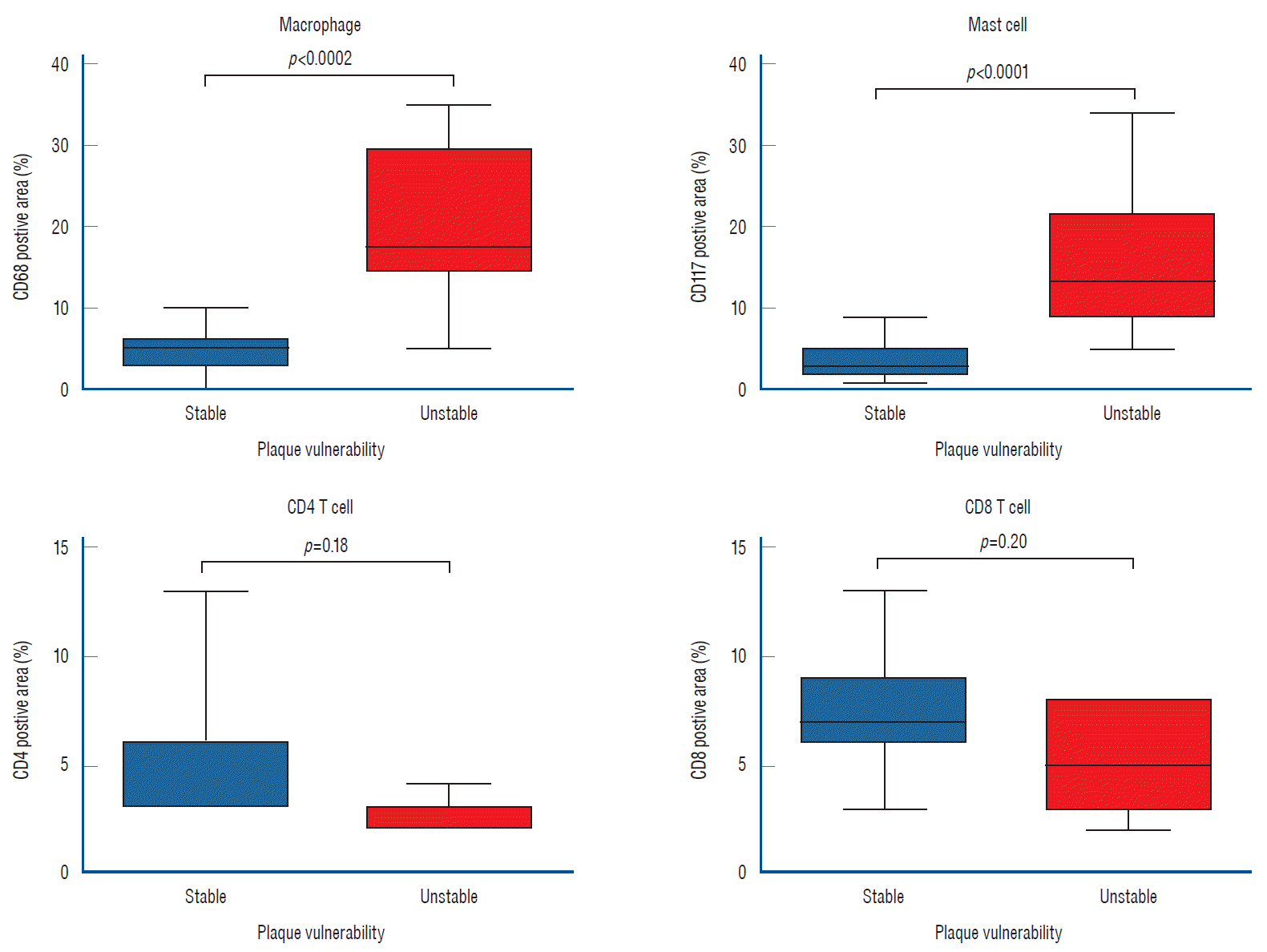
Table 1.
Clinical characteristics of patients who underwent carotid endarterectomy
| Characteristic | Value |
|---|---|
| Age in years at operation (years) | 68.5 (47–82) |
| Sex | |
| Male | 55 (79.7) |
| Female | 14 (20.3) |
| Location | |
| Right | 46 (66.7) |
| Left | 23 (33.3) |
| ICA stenosis (NASCET grade [29]) | |
| >60% | 1 (1.4) |
| >70% | 33 (47.8) |
| >80% | 17 (24.6) |
| >90% | 18 (26.2) |
| Risk factor | |
| Hypertension | 47 (68.1) |
| Diabetes | 25 (36.2) |
| None | 16 (23.1) |
| Angiographic instability | |
| Stable | 41 (59.4) |
| Unstable | 28 (40.6) |
| Vasa vasorum | |
| Vasa vasorum interna | 57 (82.6) |
| Vasa vasorum externa | 11 (16) |
| Vasa vasorum venosum | 1 (1.4) |
| ICG stagnation | |
| Yes | 42 (60.9) |
| No | 27 (39.1) |
| Plaque vulnerability | |
| Stable | 30 (43.5) |
| Unstable | 39 (56.5) |
| Postoperative complication | 5 (7.2) |
| Hyperperfusion | 1 (1.4) |
| Infraction | 2 (2.9) |
| Medical complication | 2 (2.9) |
| Outcome | |
| Good recovery | 67 (97.1) |
| Moderate disability | 2 (2.9) |
| Vasa vasorum externa | 2 (100.0) |
| Vasa vasorum interna | 0 |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download