Abstract
Treatment of chronic subdural hematoma (CSDH) is relatively straightforward, however, there is still some debate regarding the best strategy for treatment. The most practical recommendations of up to date were identified by a review of literature. The author reviewed the literature on CSDH management from the past to now to identify the best methods. Till 1970s, craniotomy was the most commonly used method. Burr hole (BH) became the most preferred method from 1980s. In 1977, twist drill (TD) craniostomy was introduced. Closed system drainage after a BH or a TD became the most frequently used surgical method. Although nonsurgical treatment is often successful, trephination has more advantages, such as rapid resolution of the symptoms and short period of hospitalization. Nonsurgical treatment is possible in asymptomatic patients with a small CSDH. For the symptomatic patients with CSDH, trephination is the treatment of choice, either by BH or TD. In gray zone between surgery and medical treatment, shared decision making can be an ideal approach. For the recurrent CSDHs, repeated trephination is still effective for patients with a low risk of recurrence. If the risk of recurrence is high, additional management would be helpful. For the refractory CSDHs, it is necessary to obliterate the subdural space.
Treatment of chronic subdural hematoma (CSDH) has improved dramatically in recent years because of advances in diagnosis and surgical techniques. However, there is still some debate regarding the best strategy for treatment [20,72,97]. There are numerous modifications or alternative methods to reduce the recurrence rate, however, the efficacy is still controversial. The author tried to find the most practical recommendations of up to date.
The author reviewed the literature on CSDH management from the past to now to identify the best methods.
There was a report that the first trephination for CSDH was made by Hulke in 19th century [94]. In the 1860s and 1870s, the most successful skull surgery in the world was done by some native surgeons in the South Pacific, the 'wise men' of Tolai tribe of New Guinea [54]. Even though the mortality rate of one of Tolai surgeons was 8 or 25%, which compared with a mortality of 75% in London teaching hospitals in late 19th century [45], no one could remember any names of the wise men. In a report of 1930 [18], the recognized treatment of CSDH was craniotomy, although it could be managed by trephination. Till 1970s, craniotomy with or without removal of the membrane was more frequently used [30] than dual cranial perforations [23] or trephination with irrigation [33]. Before cerebral angiography, it was hard to localize the hematoma. Exploratory burr-hole (BH) was an option to find the hematoma till 1960s. It looks like a ‘woodpecker surgery’ [35], however, this type of surgery might save a life. Woodpecker surgery of exploratory BHs disappeared after introduction of computerized tomographic (CT) scans in 1973 [14]. Since CSDH is consisted of mainly liquefied blood, trephination alone is usually sufficient to remove the lesion. While craniotomy requires general anesthesia and much more time to resect a bone f lap and replace it, which may cause complications. In 1975, a simple technique was reported to make a hole by an 18 G spinal needle [60]. However, making a hole with a spinal needle was not simple to be used widely. In 1977, twist drill (TD) craniostomy was published [84]. This technique was possible under local anesthesia even at the bedside. After 1980, trephination either by BH or TD became more popular than craniotomy. Closed system drainage after a BH or a TD became the most frequently used surgical treatment of CSDH [15,52,83]. To precipitate drainage, application of negative pressure to a catheter in the subdural space may result plugging by brain tissue or meninges, while larger catheters may provoke seizures [28]. In 1999, a minimal invasive device was developed to evacuate CSDH, called subdural evacuating port system (SEPS) [22], which was safe and effective as TD craniostomy [6,37,40,59,74,77]. Removal of the hematoma was possible by a TD with or without a hollow screw under local anesthesia as a bedside procedure. Although a much smaller hollow screw, 3 mm in diameter, was developed [91], the smaller does not always seem to be the better.
Systematic reviews and meta analyses suggested that the use of TD was better or at least equivalent to BHs in recurrence rates or other outcome [2,34,50,56]. Bedside TD with closed system drainage for 48 hours [34] is a safe and effective first-line management option [2].
Management of CSDH is variable (Table 1). In general, the decision to operate is influenced both by the radiographic appearance of the lesion as well as by the patient's neurological exam [20]. The spontaneous resolution or medical treatment of CSDHs has been well documented [21,36,38,63,80]. However, hospitalization ranged from 3 weeks to 42 days in some reported series and some patients eventually underwent surgery [25,89].
Inappropriate treatment was common in the earlier period, when they did not know the cause, natural history and pathophysiology of this lesion. In 1920s, lumbar puncture was the only definite non-operative treatment that had been recommended [67]. Although spontaneous resolution of CSDH was sparsely reported, the first remark about a nonsurgical treatment of CSDH was given by Ambrosetto [3] in 1962. He described four patients, who received a conservative multimodal therapy in combination with corticosteroids. In 1970, a nonsurgical treatment consisting of osmotherapy with 20% mannitol was reported [82]. The theoretical basis for osmotherapy is that mannitol and hypertonic glucose facilitate the resorption of the hematoma through the increase in the osmotic pressure of the blood. However, osmotherapy was discontinued after a failure of the mannitol therapy in a controlled clinical trial [26]. There were some reports that nonsurgical treatment with bed rest, steroids [17], mannitol [10], or a combination was successful. Successful management was also reported with some drugs inhibiting bleeding or inflammation such as angiotensin converting enzyme inhibiter [93], atorvastatin [90], and tranexamic acid [36]. Stopping the anti-coagulants or radiation was also effective to resolve the hematoma [65,68]. Nonsurgical treatment was successful for patients with age greater than 70 years, decreased cognitive level, brain atrophy, and absence of increase of intracranial pressure [63]. Favorable factors for conservative treatment were female sex, lesser midline shift, lesser thickness of the hematoma, and lesser attenuation values on CT scan [86]. However, it is unclear whether the resolution really result from the drug effect or natural course. Furthermore, nonsurgical treatment frequently requires close observation with repeated CT scans for a long time, average 40 days [38]. Surgical treatment for CSDH is quite simple to do at the bedside under local anesthesia. Compared to nonsurgical treatment, trephination by either a BH or a TD has more advantages, such as rapid resolution of the symptoms and short period of hospital admission. Avoiding surgery can’t be the goal of CSDH management.
Nonsurgical treatment may be possible in a few asymptomatic patients with a small CSDH. It requires a long apprehensive monitoring, however. Trephination is a relatively simple, safe, and effective procedure to manage this condition. Not all patients with CSDH requires surgery. In general, the decision to operate is based on the presence of symptoms and clinical or imaging signs of cerebral compression. Of course there is a gray zone between surgery and medical treatment. It may be difficult to decide a certain therapy for an asymptomatic patient with a significant amount of CSDH, or a patient with an obscure symptom and a small CSDH. Decision making may be different from the doctors [8]. In general, neurosurgeons prefer surgery while neurologists prefer medical therapy [11]. In this situation, so called shared decision making is the method of choice. Through shared decision making, clinicians can help patients understand the importance of their values and preferences in making the decisions that are best for them [7]. Doctors should provide information on the natural history, predicted prognosis, risk and benefits of a certain therapy, and so on. Although the fate of CSDH depends on the pre-morbid status, the dynamics of absorption-expansion and maturation of the neomembrane [46], however, the natural history of a certain CSDH remains unclear. Patients who have minimal neurological deficits and lesions of small size with low or isodensity and ventricular dilatation on CT have a greater chance for spontaneous resolution of their hematoma [57]. Medical therapy can be an alternative for patients with mild symptoms and frontal hematomas [31], or patients with a premorbid condition not allowing surgical evacuation of CSDH [78]. Shared decision making can be an ideal approach to clinician–patient decision making, improve the quality of medical decisions, and reduce costs [62]. A scoring system [42] can be a useful tool to predict outcome and result of surgical intervention.
The most commonly used surgical methods are TD craniostomy, BH trephination, and craniotomy. They have their own advantages and disadvantages (Table 2). Openings of the skull up to a diameter of 5 mm were categorised as TD craniostomy, openings of up to 30 mm as BH craniostomy, and larger openings as craniotomy [95].
It is difficult to identify the most effective surgical method for CSDH. A study by decision analysis reported that the BH is the most efficient choice for surgical drainage of uncomplicated CSDH [48]. An evidence based review [2] revealed no difference in the cure rates and mortality among the three techniques. The authors concluded that BH provided the best cure-to-complication ratio, since TD had a significantly higher rate of recurrence and craniotomy had higher morbidity. A systematic review and meta-analysis with 34829 patients revealed no significant difference in the rate of cure, recurrence, morbidity, or mortality between TD and BH [2]. They recommended bedside TD as a relatively safe and effective first-line management option. Although there are diverse practices in detail, vast majority of CSDH can be treated either by TD or BH [75]. Most surgeons would choose the familiar method [47]. As the first-line treatment option, TD is the best, since it can be done at the bedside under local anesthesia, which saves health cost and eliminate perioperative risks related to general anesthesia. BH may be an alternative of TD. Since craniotomy was found to result in a higher complications, it should be reserved for solid, organized or calcified hematomas. Actually in a retrospective study, craniotomy result longer operation time, longer hospital stay with more complications and higher cost. Craniotomy seems to be necessary for the organized or calcified CSDH, when the CSDH is manifested by seizure or hemiparesis. Headache alone is hard to be a relevant symptom of the longstanding organized CSDH, although it is the third most frequent symptom of CSDH [98]. In calcified CSDHs, observation is recommended for asymptomatic patients or patients with a headache alone. Although Markwalder had recommended craniotomy for recurrent CSDH, solid hematoma, or failure of the brain re-expansion [52], solid consistency of the hematoma might be the only indication of craniotomy. Most recurrent subdural hematomas can be managed successfully via BH with closed-system drainage [19]. Refractory hematomas which was recurrent more than twice, may require subduroperitoneal shunt, a reservoir with serial tapping, or middle meningeal artery embolization. Even in refractory CSDHs, subduroperitoneal shunt or middle meningeal artery embolization is less invasive than craniotomy. Some authors recommended endoscopic removal [13,29], continuous postoperative irrigation and drainage [96], or injection of isotonic fluid into the ventricular space to promote brain re-expansion for refractory hematomas [70], the impact of these measures is controversial.
The method of anesthesia depends on the method of surgery. General anesthesia is necessary for long operation with large skin incision. Local anesthesia is usually sufficient with percutaneous drainage [2]. In general, general anesthesia may cause cardiovascular or respiratory complications, while local anesthesia may reduce the duration of drainage or hospital stay. However, local anesthesia is safe for cooperative patients or patients with high risk systemic disorders which prevent general anesthesia. Anesthetic method also depends on the patient condition.
There was a report [85] that one BH only was associated with a significantly higher recurrence rate, longer hospital stay and higher infection rate. A meta-analysis suggested that single BH is as good as double BHs [9]. Another BH is usually not necessary.
Irrigation of the hematoma cavity may dilute anticoagulating substances such as fibrin degradation product, which may reduce the chance of recurrence. However, air intrusion during irrigation may cause tension pneumocephalus, headache, or recurrence [41]. Irrigation itself may cause cortical injury or bleeding [96]. It is not necessary for all CSDHs, although it may be helpful when the hematoma has solid mass [97].
Removal of the membranes after craniotomy is to eradicate the bleeding source and to facilitate re-expansion of the brain. Membranectomy was often recommended when there was solid hematomas with various bleeding foci, multilayer loculations within the hematoma, and excessive formation of a solid membranes [39]. However, membranectomy carries a distinctly higher risk of rebleeding, especially membranes surrounding the bridge veins and the area of tenacious adherence between inner membrane and arachnoid surface [44,71]. Even in hematomas with multiple septations, it is not necessary to remove the membrane. The result of surgery and the rate of recurrence was not influenced by the membranectomy or partial tearing of the membranes. Aggressive removal of membranes may cause cortical injury, which evokes postoperative convulsive disorder [71].
Drain is very useful to remove the remained hematoma slowly. However, prolonged placement of the drain may raise the infection rate [53]. Drainage for 48 hours has the advantage of reducing recurrence without increasing complications [1,43,50,75]. Postoperative position whether supine or sitting, did not show any differences in the recurrence rate [58]. However, early mobilization is recommended to avoid complications related prolonged recumbence. Postoperative physical activity is usually not limited in patients with chronic subdural hematoma. However, it would be desirable to avoid a certain exercise, which may cause shaking of the head. Postoperative CT scan is often useful to confirm the result of surgery, however, routine CT around 4–6 weeks after surgery is not recommended [64].
Subdural tapping is useful in infants [4]. It can be available in children or even in adults. If the skull is hard to penetrate by the needle, a path can be made by a hand drill [69]. The endoscopic treatment of septated or organized CSDH represents a minimally invasive alternative to craniotomy especially for the internal membranectomy [13,71]. Endoscopic management is not popular since fibrin septa or thick inner membranes hindering drainage represents less than 5% of all CSDH. Actually, membranectomy is seldom required for brain re-expansion. Thrombolytic agent is often useful to remove partially resolved hematomas [5].
Mini-craniotomy, the diameter of the bone flap remains limited to 3–4 cm, may have superior visualization, while the invasiveness and complication rate are equal to those of BH [87]. There was a report of lower recurrences with mini-craniotomy, however, this technique is not recommended since the removal of the membranes doesn’t precipitate the brain re-expansion.
Subduroperitoneal shunts, though rarely used, may be a viable option in the treatment of CSDH, especially in refractory cases [88]. However, the shunt requires a device implanted into the peritoneal cavity, which needs general anesthesia. Furthermore it requires consistent monitoring and often fail. Previously, it was useful for pediatric CSDHs, or older patients with recurrent or thick membranes [66,88]. This method may be reserved for refractory or cases of at least three times recurrence. There are some modified techniques such as implantation of a reservoir [76], continuous subgaleal suction drainage [24], or a T-tube drainage [51]. Although they may reduce additional operations [43], these modifications failed to get popularity.
Recently embolization of the middle meningeal artery (EMMA) with or without trephination was reported as an effective method [49]. Although EMMA interrupts the blood supply to the outer membrane and thus prevents hematoma enlargement, it often fails to prevent recurrences [16]. Sufficient subdural space is the key factor for development of CSDH. Theoretically, elimination of the subdural space is fundamental than prevention of hematoma enlargement to prevent the recurrence. To reduce the subdural space, lumbar or ventricular injection at the time of operation and/or afterward is effective and safe for immediate cerebral re-expansion [27]. Since trephination with closed system drainage is easy, safe and effective, lumbar or ventricular injection is seldom used. Refractory CSDHs may require complete obliteration of the subdural hematoma cavity by reducing the dura or fibrin glue injection [61,92]. When there is a macrocephaly from expanding CSDH, reduction cranioplasty may become the last choice [55,81]. Fortunately, such cases are extremely rare.
Up to date, trephination either by BH or TD is the treatment of choice for symptomatic patients (Fig. 1). Craniotomy should be reserved only for symptomatic organized or solid hematomas. For the recurrent CSDHs, if the risk of recurrence is low, still repeated trephination is simple and effective. If the risk of recurrence is high, additional management such as EMMA may reduce the chance of recurrence. Oslo grading system [79] seems to be very useful to predict the risk of recurrence. For the refractory CSDHs, it is necessary to obliterate the subdural space by lumbar or ventricular injection, fibrin glue injection or reducing the dura. Subduroperitoneal shunts can be the last choice for refractory CSDHs. If there is a new easy and safe therapy, we should change this algorithm.
Nonsurgical treatment is possible in asymptomatic patients with a small CSDH. For the symptomatic patients with CSDH, trephination is the treatment of choice, either by BH or TD. In gray zone between surgery and medical treatment, shared decision making can be an ideal approach. For the recurrent CSDHs, repeated trephination is still effective for patients with a low risk of recurrence. If the risk of recurrence is high, additional management would be helpful. For the refractory CSDHs, it is necessary to obliterate the subdural space.
References
1. Alcalá-Cerra G, Young AM, Moscote-Salazar LR, Paternina-Caicedo Á. Efficacy and safety of subdural drains after burr-hole evacuation of chronic subdural hematomas: systematic review and meta-analysis of randomized controlled trials. World Neurosurg. 82:1148–1157. 2014.

2. Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, Yarascavitch B, et al. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg. 259:449–457. 2014.
3. Ambrosetto C. Post-traumatic subdural hematoma. Further observations on nonsurgical treatment. Arch Neurol. 6:287–292. 1962.
4. Aoki N. Chronic subdural hematoma in infancy. Clinical analysis of 30 cases in the CT era. J Neurosurg. 73:201–205. 1990.
5. Arginteanu MS, Byun H, King W. Treatment of a recurrent subdural hematoma using urokinase. J Neurotrauma. 16:1235–1239. 1999.

6. Asfora WT, Schwebach L. A modified technique to treat chronic and subacute subdural hematoma: technical note. Surg Neurol. 59:329–332. discussion 332. 2003.

7. Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 366:780–781. 2012.

8. Baschera D, Tosic L, Westermann L, Oberle J, Alfieri A. Treatment standards for chronic subdural hematoma: results from a survey in Austrian, German and Swiss neurosurgical units. World Neurosurg. 116:e983–e995. 2018.

9. Belkhair S, Pickett G. One versus double burr holes for treating chronic subdural hematoma meta-analysis. Can J Neurol Sci. 40:56–60. 2013.

10. Bender MB, Christoff N. Nonsurgical treatment of subdural hematomas. Arch Neurol. 31:73–79. 1974.

11. Berghauser Pont LM, Dippel DW, Verweij BH, Dirven CM, Dammers R. Ambivalence among neurologists and neurosurgeons on the treatment of chronic subdural hematoma: a national survey. Acta Neurol Belg. 113:55–59. 2013.

12. Berghauser Pont LM, Dirven CM, Dippel DW, Verweij BH, Dammers R. The role of corticosteroids in the management of chronic subdural hematoma: a systematic review. Eur J Neurol. 19:1397–1403. 2012.

13. Berhouma M, Jacquesson T, Jouanneau E. The minimally invasive endoscopic management of septated chronic subdural hematomas: surgical technique. Acta Neurochir (Wien). 156:2359–2362. 2014.

14. Bleck TP. Historical aspects of critical care and the nervous system. Crit Care Clin. 25:153–164. ix. 2009.

15. Cenic A, Bhandari M, Reddy K. Management of chronic subdural hematoma: a national survey and literature review. Can J Neurol Sci. 32:501–506. 2005.

16. Chihara H, Imamura H, Ogura T, Adachi H, Imai Y, Sakai N. Recurrence of a refractory chronic subdural hematoma after middle meningeal artery embolization that required craniotomy. NMC Case Rep J. 1:1–5. 2014.

17. Delgado-López PD, Martín-Velasco V, Castilla-Díez JM, Rodríguez-Salazar A, Galacho-Harriero AM, Fernández-Arconada O. Dexamethasone treatment in chronic subdural haematoma. Neurocirugia. 20:346–359. 2009.

18. D’Errico AP, German WJ. Chronic subdural hematoma. Yale J Biol Med. 3:11–20. 1930.
19. Desai VR, Scranton RA, Britz GW. Management of recurrent subdural hematomas. Neurosurg Clin N Am. 28:279–286. 2017.

20. Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Anderson KN, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev. 35:155–169. discussion 169. 2012.

21. Emich S, Richling B, McCoy MR, Al-Schameri RA, Ling F, Sun L, et al. The efficacy of dexamethasone on reduction in the reoperation rate of chronic subdural hematoma--the DRESH study: straightforward study protocol for a randomized controlled trial. Trials. 15:6. 2014.
22. Emonds N, Hassler WE. New device to treat chronic subdural hematoma--hollow screw. Neurol Res. 21:77–78. 1999.

24. Gazzeri R, Galarza M, Neroni M, Canova A, Refice GM, Esposito S. Continuous subgaleal suction drainage for the treatment of chronic subdural haematoma. Acta Neurochir (Wien). 149:487–493. discussion 493. 2007.

25. Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 107:223–229. 2005.

26. Gjerris F, Schmidt K. Chronic subdural hematoma. Surgery or mannitol treatment. J Neurosurg. 40:639–642. 1974.
27. Grisoli F, Graziani N, Peragut JC, Vincentelli F, Fabrizi AP, Caruso G, et al. Perioperative lumbar injection of Ringer’s lactate solution in chronic subdural hematomas: a series of 100 cases. Neurosurgery. 23:616–621. 1988.

28. Gurelik M, Aslan A, Gurelik B, Ozum U, Karadag Ö, Kars HZ. A safe and effective method for treatment of chronic subdural haematoma. Can J Neurol Sci. 34:84–87. 2007.

29. Hellwig D, Kuhn TJ, Bauer BL, List-Hellwig E. Endoscopic treatment of septated chronic subdural hematoma. Surg Neurol. 45:272–277. 1996.

30. Hirakawa K, Hashizume K, Fuchinoue T, Takahashi H, Nomura K. Statistical analysis of chronic subdural hematoma in 309 adult cases. Neurol Med Chir (Tokyo). 12:71–83. 1972.

31. Horikoshi T, Naganuma H, Fukasawa I, Uchida M, Nukui H. Computed tomography characteristics suggestive of spontaneous resolution of chronic subdural hematoma. Neurol Med Chir (Tokyo). 38:527–532. discussion 532-523. 1998.

32. Horn EM, Feiz-Erfan I, Bristol RE, Spetzler RF, Harrington TR. Bedside twist drill craniostomy for chronic subdural hematoma: a comparative study. Surg Neurol. 65:150–153. discussion 153-154. 2006.

33. Horrax G, Poppen JL. The frequency, recognition and treatment of chronic subdural hematomas. N Engl J Med. 216:381–385. 1937.

34. Ivamoto HS, Lemos HP Jr, Atallah AN. Surgical treatments for chronic subdural hematomas: a comprehensive systematic review. World Neurosurg. 86:399–418. 2016.

35. Jamieson KG. Extradural and subdural hematomas. Changing patterns and requirements of treatment in Australia. J Neurosurg. 33:632–635. 1970.
36. Kageyama H, Toyooka T, Tsuzuki N, Oka K. Nonsurgical treatment of chronic subdural hematoma with tranexamic acid. J Neurosurg. 119:332–337. 2013.

37. Kenning TJ, Dalfino JC, German JW, Drazin D, Adamo MA. Analysis of the subdural evacuating port system for the treatment of subacute and chronic subdural hematomas. J Neurosurg. 113:1004–1010. 2010.

38. Kim HC, Ko JH, Yoo DS, Lee SK. Spontaneous resolution of chronic subdural hematoma: close observation as a treatment strategy. J Korean Neurosurg Soc. 59:628–636. 2016.

39. Kim JH, Kang DS, Kim JH, Kong MH, Song KY. Chronic subdural hematoma treated by small or large craniotomy with membranectomy as the initial treatment. J Korean Neurosurg Soc. 50:103–108. 2011.

40. Krieg SM, Aldinger F, Stoffel M, Meyer B, Kreutzer J. Minimally invasive decompression of chronic subdural haematomas using hollow screws: efficacy and safety in a consecutive series of 320 cases. Acta Neurochir (Wien). 154:699–705. discussion 705. 2012.

41. Kuroki T, Katsume M, Harada N, Yamazaki T, Aoki K, Takasu N. Strict closed-system drainage for treating chronic subdural haematoma. Acta Neurochir (Wien). 143:1041–1044. 2001.

42. Kwon CS, Al-Awar O, Richards O, Izu A, Lengvenis G. Predicting prognosis of patients with chronic subdural hematoma: a new scoring system. World Neurosurg. 109:e707–e714. 2018.

43. Laumer R, Schramm J, Leykauf K. Implantation of a reservoir for recurrent subdural hematoma drainage. Neurosurgery. 25:991–996. 1989.

44. Lee JY, Ebel H, Ernestus RI, Klug N. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients: is membranectomy necessary? Surg Neurol. 61:523–527. discussion 527-528. 2004.

46. Lee KS. Natural history of chronic subdural haematoma. Brain Inj. 18:351–358. 2004.
47. Lee SJ, Hwang SC, Im SB. Twist-drill or burr hole craniostomy for draining chronic subdural hematomas: how to choose it for chronic subdural hematoma drainage. Korean J Neurotrauma. 12:107–111. 2016.

48. Lega BC, Danish SF, Malhotra NR, Sonnad SS, Stein SC. Choosing the best operation for chronic subdural hematoma: a decision analysis. J Neurosurg. 113:615–621. 2010.

49. Link TW, Schwarz JT, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for recurrent chronic subdural hematoma: a case series. World Neurosurg. 118:e570–e574. 2018.

50. Liu W, Bakker NA, Groen RJ. Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures. J Neurosurg. 121:665–673. 2014.

51. Lu W, Wang H, Wu T, Sheng X, Ding Z, Xu G. Burr-hole Craniostomy with T-tube drainage as surgical treatment for chronic subdural hematoma. World Neurosurg. 115:e756–e760. 2018.

53. Markwalder TM, Seiler RW. Chronic subdural hematomas: to drain or not to drain? Neurosurgery. 16:185–188. 1985.

55. Matsui T, Tsutsumi K, Kaizu H, Asano T. Reduction cranioplasty for craniocerebral disproportion due to chronic subdural hematoma in infants. A technical report. Neurol Res. 23:67–71. 2001.

56. Montano N, Ioannoni E, Caricato A, Olivi A. Survey of available literature and meta-analyses on chronic subdural hematoma. Int J Med Rev. 3:497–500. 2018.
57. Naganuma H, Fukamachi A, Kawakami M, Misumi S, Nakajima H, Wakao T. Spontaneous resolution of chronic subdural hematomas. Neurosurgery. 19:794–798. 1986.

58. Nakajima H, Yasui T, Nishikawa M, Kishi H, Kan M. The role of postoperative patient posture in the recurrence of chronic subdural hematoma: a prospective randomized trial. Surg Neurol. 58:385–387. discussion 387. 2002.

59. Neal MT, Hsu W, Urban JE, Angelo NM, Sweasey TA, Branch CL Jr. The subdural evacuation port system: outcomes from a single institution experience and predictors of success. Clin Neurol Neurosurg. 115:658–664. 2013.

60. Negrón RA, Tirado G, Zapater C. Simple bedside technique for evacuating chronic subdural hematomas. Technical note. J Neurosurg. 42:609–611. 1975.
61. Oku Y, Takimoto N, Yamamoto K, Onishi T. Trial of a new operative method for recurrent chronic subdural hematoma. J Neurosurg. 61:269–272. 1984.

62. Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med. 368:6–8. 2013.

63. Parlato C, Guarracino A, Moraci A. Spontaneous resolution of chronic subdural hematoma. Surg Neurol. 53:312–315. discussion 315-317. 2000.

64. Pedersen CB, Sundbye F, Poulsen FR. No value of routine brain computed tomography 6 weeks after evacuation of chronic subdural hematoma. Surg J (N Y). 3:e174–e176. 2017.

65. Prevedi G. Roentgen-therapy of subacute and chronic subdural hematomas controlled by carotid angiography. Radiol Med. 52:436–447. 1966.
66. Probst C. Peritoneal drainage of chronic subdural hematomas in older patients. J Neurosurg. 68:908–911. 1988.

67. Putnam TJ, Cushing H. Chronic subdural hematoma: its pathology, its relation to pachymeningitis hemorrhagica and its surgical treatment. Archi Surg. 11:329–393. 1925.
68. Ratre S, Yadav Y, Choudhary S, Parihar V. Spontaneous resolution of chronic subdural haematoma in a patient receiving anticoagulant therapy. J Assoc Physicians India. 63:79–80. 2015.
69. Reinges MH, Hasselberg I, Rohde V, Küker W, Gilsbach JM. Prospective analysis of bedside percutaneous subdural tapping for the treatment of chronic subdural haematoma in adults. J Neurol Neurosurg Psychiatry. 69:40–47. 2000.

70. Robinson RG. Chronic subdural hematoma: surgical management in 133 patients. J Neurosurg. 61:263–268. 1984.

71. Rocchi G, Caroli E, Salvati M, Delfini R. Membranectomy in organized chronic subdural hematomas: indications and technical notes. Surg Neurol. 67:374–380. discussion 380. 2007.

72. Rovlias A, Theodoropoulos S, Papoutsakis D. Chronic subdural hematoma: surgical management and outcome in 986 cases: a classification and regression tree approach. Surg Neurol Int. 6:127. 2015.

73. Rudiger A, Ronsdorf A, Merlo A, Zimmerli W. Dexamethasone treatment of a patient with large bilateral chronic subdural haematomata. Swiss Med Wkly. 131:387. 2001.
74. Rughani AI, Lin C, Dumont TM, Penar PL, Horgan MA, Tranmer BI. A case-comparison study of the subdural evacuating port system in treating chronic subdural hematomas. J Neurosurg. 113:609–614. 2010.

75. Santarius T, Lawton R, Kirkpatrick PJ, Hutchinson PJ. The management of primary chronic subdural haematoma: a questionnaire survey of practice in the United Kingdom and the Republic of Ireland. Br J Neurosurg. 22:529–534. 2008.

76. Sato M, Iwatsuki K, Akiyama C, Kumura E, Yoshimine T. Implantation of a reservoir for refractory chronic subdural hematoma. Neurosurgery. 48:1297–1301. 2001.

77. Singla A, Jacobsen WP, Yusupov IR, Carter DA. Subdural evacuating port system (SEPS)--minimally invasive approach to the management of chronic/subacute subdural hematomas. Clin Neurol Neurosurg. 115:425–431. 2013.

78. Soleman J, Taussky P, Fandino J, Muroi C. Evidence-based treatment of chronic subdural hematoma in Sadaka DF (ed) : Traumatic Brain Injury, ed 1. London: InTech;2014. p. 249–282.
79. Stanišic M, Pripp AH. A reliable grading system for prediction of chronic subdural hematoma recurrence requiring reoperation after initial burrhole surgery. Neurosurgery. 81:752–760. 2017.

80. Sun TF, Boet R, Poon WS. Non-surgical primary treatment of chronic subdural haematoma: preliminary results of using dexamethasone. Br J Neurosurg. 19:327–333. 2005.

81. Sundine MJ, Wirth GA, Brenner KA, Loudon WG, Muhonen MG, Greene CS, et al. Cranial vault reduction cranioplasty in children with hydrocephalic macrocephaly. J Craniofac Surg. 17:645–655. 2006.

82. Suzuki J, Takaku A. Nonsurgical treatment of chronic subdural hematoma. J Neurosurg. 33:548–553. 1970.

83. Suzuki K, Sugita K, Akai T, Takahata T, Sonobe M, Takahashi S. Treatment of chronic subdural hematoma by closed-system drainage without irrigation. Surg Neurol. 50:231–234. 1998.

84. Tabaddor K, Shulman K. Definitive treatment of chronic subdural hematoma by twist-drill craniostomy and closed-system drainage. J Neurosurg. 46:220–226. 1977.

85. Taussky P, Fandino J, Landolt H. Number of burr holes as independent predictor of postoperative recurrence in chronic subdural haematoma. Br J Neurosurg. 22:279–282. 2008.

86. Thotakura AK, Marabathina NR. Nonsurgical treatment of chronic subdural hematoma with steroids. World Neurosurg. 84:1968–1972. 2015.

87. Van Der Veken J, Duerinck J, Buyl R, Van Rompaey K, Herregodts P, D’Haens J. Mini-craniotomy as the primary surgical intervention for the treatment of chronic subdural hematoma--a retrospective analysis. Acta Neurochir (Wien). 156:981–987. 2014.

88. Vinchon M, Noulé N, Soto-Ares G, Dhellemmes P. Subduroperitoneal drainage for subdural hematomas in infants: results in 244 cases. J Neurosurg. 95:249–255. 2001.

89. Voelker JL. Nonoperative treatment of chronic subdural hematoma. Neurosurg Clin N Am. 11:507–513. 2000.

90. Wang D, Li T, Tian Y, Wang S, Jin C, Wei H, et al. Effects of atorvastatin on chronic subdural hematoma: a preliminary report from three medical centers. J Neurol Sci. 336:237–242. 2014.

91. Wang QF, Cheng C, You C. A new modified twist drill craniostomy using a novel device to evacuate chronic subdural hematoma. Medicine (Baltimore). 95:e3036. 2016.

92. Watanabe S, Amagasaki K, Shono N, Nakaguchi H. Fibrin glue injection into the hematoma cavity for refractory chronic subdural hematoma: a case report. Surg Neurol Int. 7(Suppl 37):S876–S879. 2016.

93. Weigel R, Hohenstein A, Schlickum L, Weiss C, Schilling L. Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma possibly by an antiangiogenic mechanism. Neurosurgery. 61:788–792. discussion 792-793. 2007.

94. Weigel R, Krauss JK, Schmiedek P. Concepts of neurosurgical management of chronic subdural haematoma: historical perspectives. Br J Neurosurg. 18:8–18. 2004.

95. Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 74:937–943. 2003.

96. Xu C, Chen S, Yuan L, Jing Y. Burr-hole irrigation with closed-system drainage for the treatment of chronic subdural hematoma: a meta-analysis. Neurol Med Chir (Tokyo). 56:62–68. 2016.

97. Xu CS, Lu M, Liu LY, Yao MY, Cheng GL, Tian XY, et al. Chronic subdural hematoma management: clarifying the definitions of outcome measures to better understand treatment efficacy - a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 21:809–818. 2017.
Fig. 1.
Algorithm for practical management of chronic subdural hematoma. EMMA : embolization of middle meningeal artery, SDSO : subdural space obliteration, SDP : subduroperitoneal.
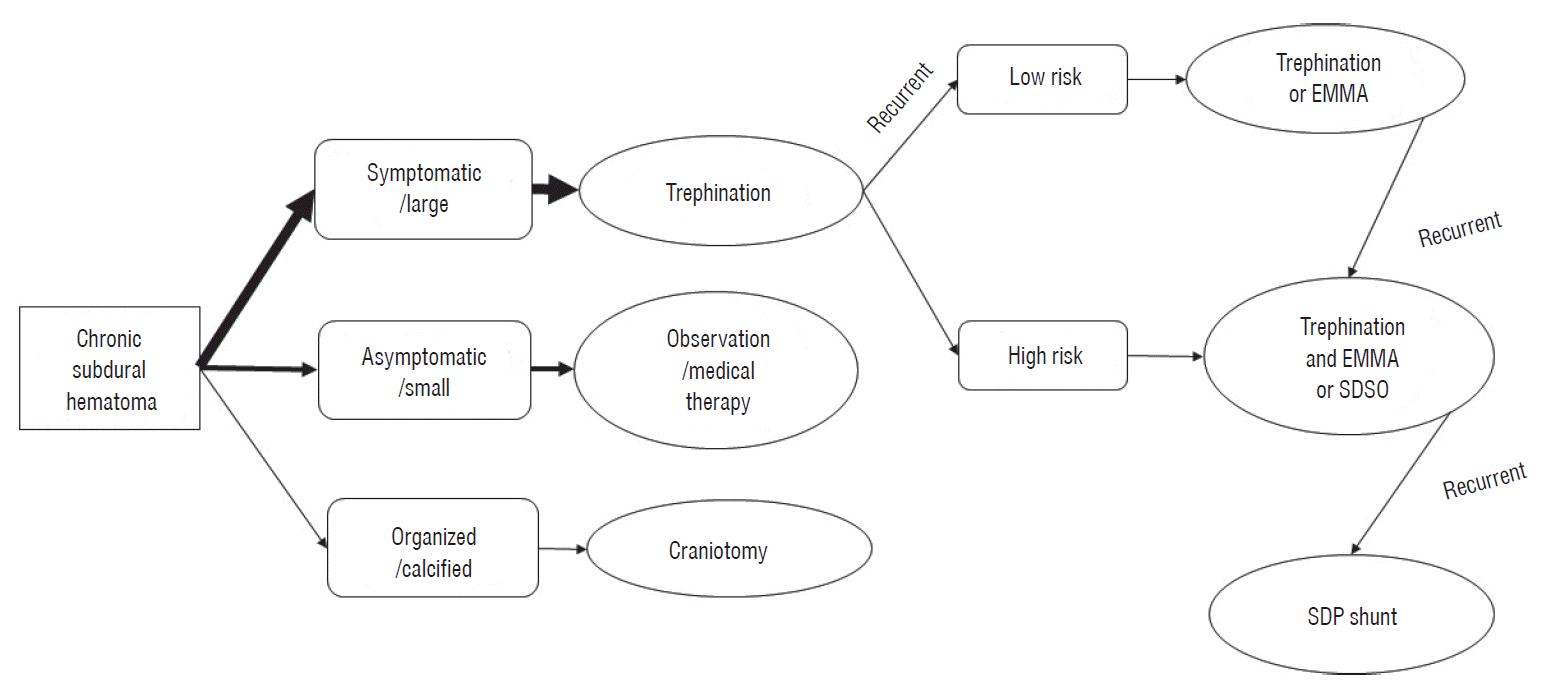
Table 1.
Variable methods of treatment for chronic subdural hematomas
| Group | Value |
|---|---|
| Non-surgical | Bed rest [31], steroids [12,73,80], mannitol [44], tranexamic acid [24,36] |
| Minimally invasive surgery | Twisted drill craniostomy [32], burr hole trephination, endoscope [13,29] |
| Traditional surgery | Craniotomy, craniectomy |
| Uncommon methods | Subdural tap, subduroperitoneal shunt, subgaleal reservoir, reduction cranioplasty, middle meningeal artery embolization |
Table 2.
Characteristics of three operative methods




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download