INTRODUCTION
MATERIALS AND METHODS
Animals
Experimental design
6-HD induced lesions
Surgery and microdialysis procedures
DBS
L-glutamate measurement
TH immunohistochemistry
Cresyl violet staining
Statistical analysis
RESULTS
Influence of EPN stimulation on glutamate levels in striatum
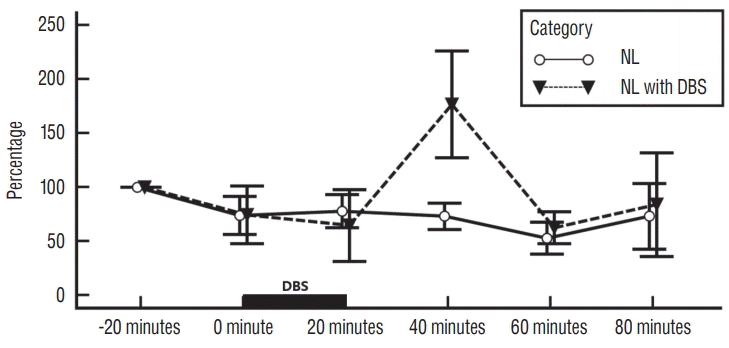 | Fig. 1.Influence of EPN stimulation on striatal glutamate levels in no-lesioned rats. Each point represents the mean±standard error from six rats per category : no-lesioned rats without deep brain stimulation (NL category) and no-lesioned rats with deep brain stimulation (NL with DBS category). DBS : deep brain stimulation, NL : no-lesioned, EPN : entopeduncular nucleus. |
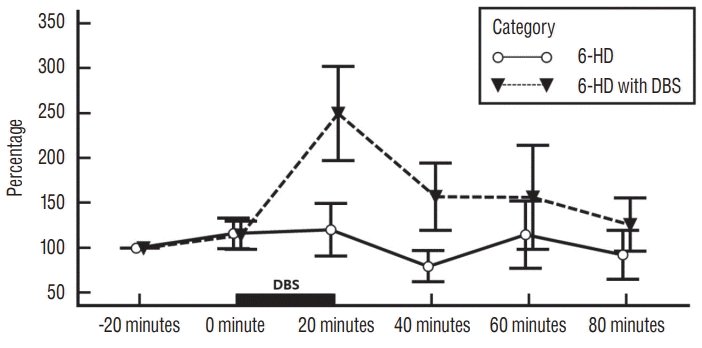 | Fig. 2.Influence of EPN stimulation on striatal glutamate levels in 6-HD lesioned rats. Each point represents the mean±standard error from six rats per category : 6-HD lesioned rats without DBS (6-HD category) and 6-HD lesioned rats with deep brain stimulation (6-HD with DBS category). DBS : deep brain stimulation, 6-HD : 6-hydroxydopamine, EPN : entopeduncular nucleus. |
Influence of EPN stimulation on TH-immunoreactivity in striatum and SN
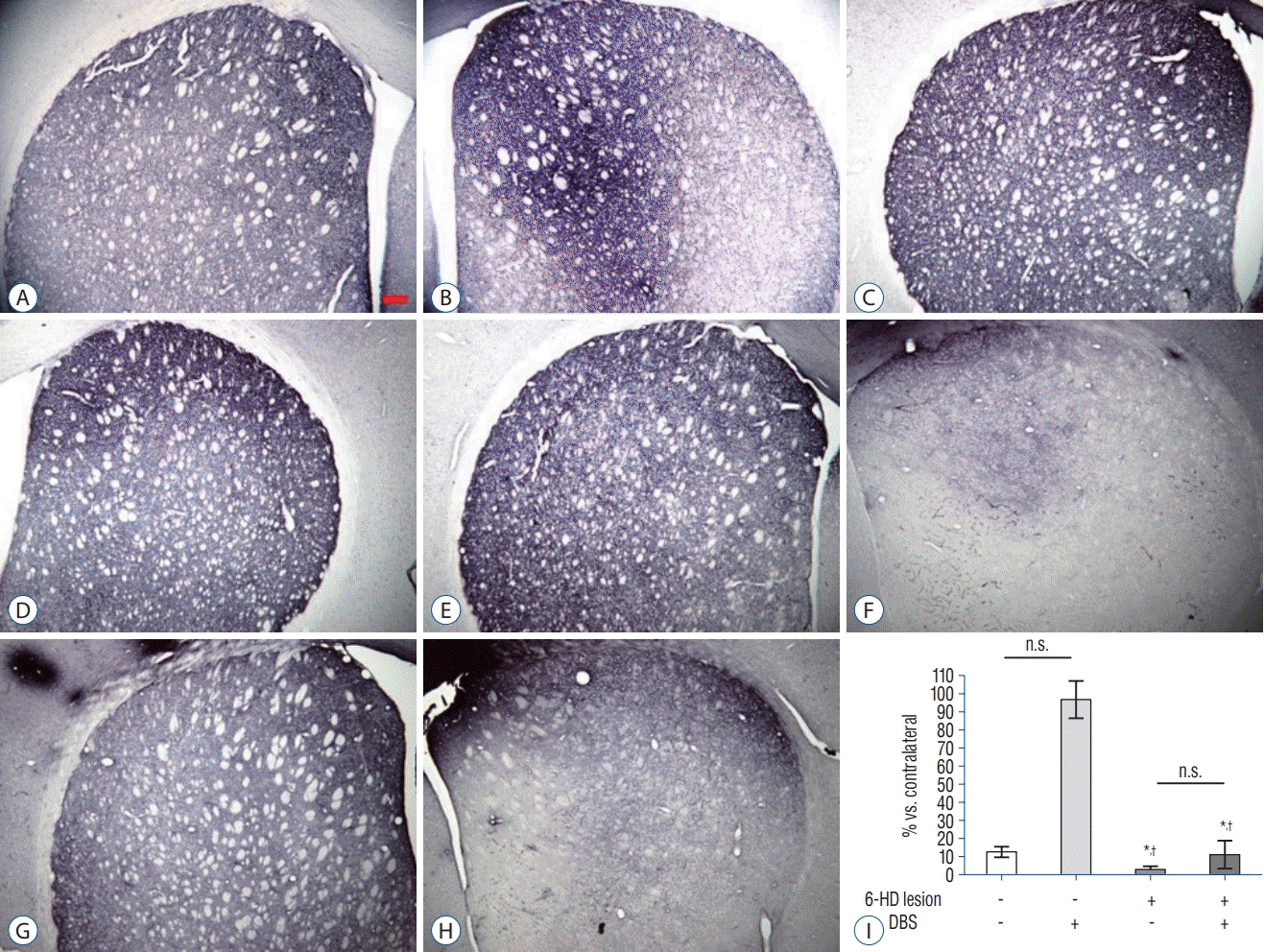 | Fig. 3.TH-immunoreactivity in the Striatum. Representative images showed TH-immunoreactivity in the striatum of the ipsilateral (A, C, E, G) and contralateral (B, D, F, H) sides. Scale bar=200 μm. A and B : NL category. C and D : NL with DBS category. E and F : 6-HD category. G and H : 6-HD with DBS category. Mean (±standard error of mean) quantification (I) of TH expression showed significant decrease in both 6-HD and 6-HD with DBS categories compared to NL categories (n=5–6 per groups). *p<0.001 versus NL category. † p<0.001 versus NL with DBS category, ANOVA. n.s. : no significance, 6-HD : 6-hydroxydopamine, DBS : deep brain stimulation, TH : tyrosine hydroxylase, NL : no-lesioned, ANOVA : analysis of variance. |
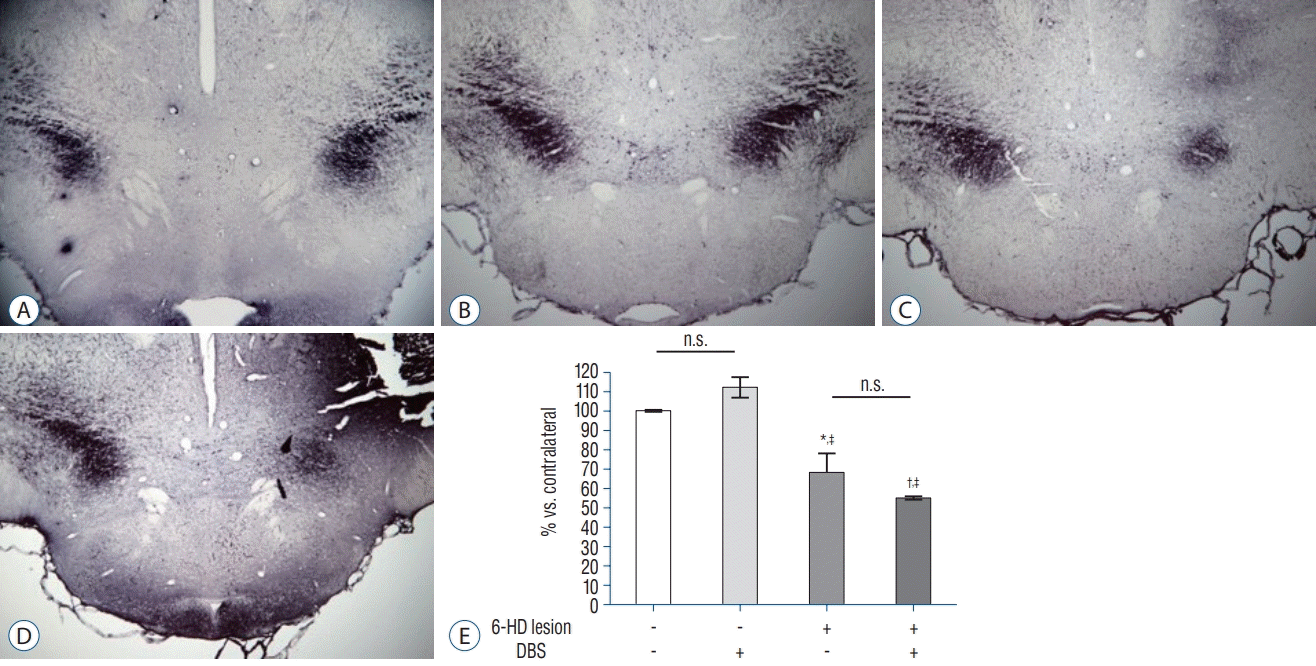 | Fig. 4.TH-immunoreactivity in the SN. Representative images showed TH-immunoreactivity in the SN. Left side is ipsilateral part and right side is contralateral part of the SN. Scale bar=200 μm. A : NL category. B : NL with DBS category. C : 6-HD category. D : 6-HD with DBS category. Mean (±standard error of mean) quantification (E) of TH expression showed significant decrease in both 6-HD category and 6-HD with DBS category compared to NL categories (n=4–6 per groups). *p<0.01 and † p <0.001 versus NL category. ‡ p<0.001 versus NL with DBS category, ANOVA. n.s. : no significance, 6-HD : 6-hydroxydopamine, DBS : deep brain stimulation, SN : substantia nigra, TH : tyrosine hydroxylase, NL : no-lesioned, ANOVA : analysis of variance. |
Histologic verification
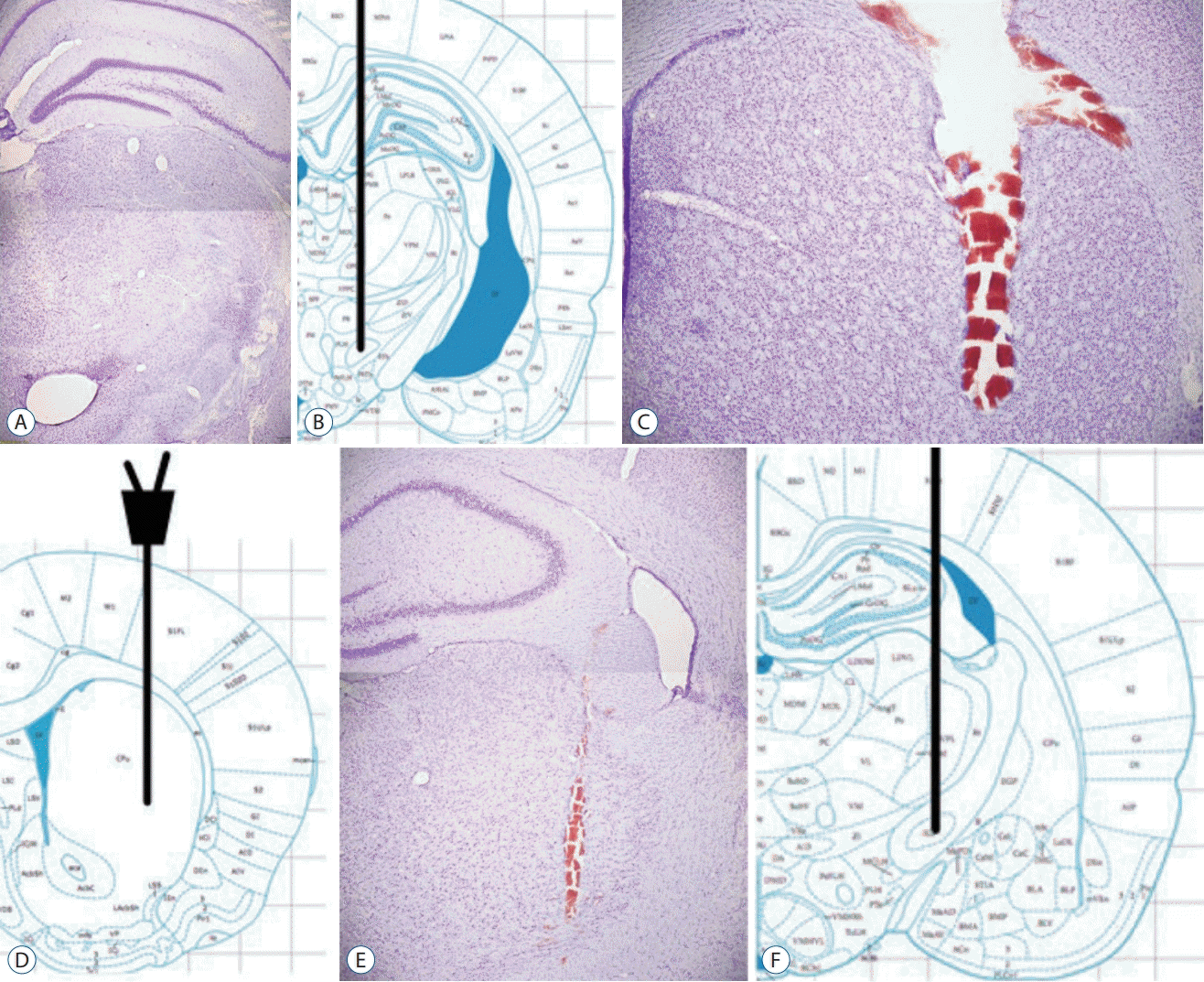 | Fig. 5.Histological verification. The stereotaxic atlas from Paxinos [23] shows the location of MFB (B), dorsal striatum (D) and EPN (F). Representation of coronal sections verifies injection site of 6-hydroxydopamine in the MFB (A), position of the probe in the striatum (C) and the electrode in the EPN (E). Scale bars=200 μm. MFB : medial forebrain bundle, EPN : entopeduncular nucleus. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download