1. Aguirre D, Nieto K, Lazos M, Peña YR, Palma I, Kofman-Alfaro S, et al. Extragonadal germ cell tumors are often associated with Klinefelter syndrome. Hum Pathol. 37:477–480. 2006.

2. Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer. 91:355–358. 2004.

3. Barbaric I, Harrison NJ. Rediscovering pluripotency: from teratocarcinomas to embryonic stem cells. Cardiff, 10-12 October 2011. Int J Dev Biol. 56:197–206. 2012.

4. Biermann K, Klingmuller D, Koch A, Pietsch T, Schorle H, Büttner R, et al. Diagnostic value of markers M2A, OCT3/4, AP-2gamma, PLAP and c-KIT in the detection of extragonadal seminomas. Histopathology. 49:290–297. 2006.

5. Calaminus G, Bamberg M, Jurgens H, Kortmann RD, Sörensen N, Wiestler OD, et al. Impact of surgery, chemotherapy and irradiation on long term outcome of intracranial malignant non-germinomatous germ cell tumors: results of the German Cooperative Trial MAKEI 89. Klin Padiatr. 216:141–149. 2004.

6. Calaminus G, Frappaz D, Kortmann RD, Krefeld B, Saran F, Pietsch T, et al. Outcome of patients with intracranial non-germinomatous germ cell tumors-lessons from the SIOP-CNS-GCT-96 trial. Neuro Oncol. 19:1661–1672. 2017.

7. Calaminus G, Kortmann R, Worch J, Nicholson JC, Alapetite C, Garrè ML, et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 15:788–796. 2013.

8. Cheng S, Kilday JP, Laperriere N, Janzen L, Drake J, Bouffet E, et al. Outcomes of children with central nervous system germinoma treated with multi-agent chemotherapy followed by reduced radiation. J Neurooncol. 127:173–180. 2016.

9. Cho J, Choi JU, Kim DS, Suh CO. Low-dose craniospinal irradiation as a definitive treatment for intracranial germinoma. Radiother Oncol. 91:75–79. 2009.

10. de Jong J, Stoop H, Gillis AJ, van Gurp RJ, van de Geijn GJ, de Boer M, et al. Differential expression of SOX17 and SOX2 in germ cells and stem cells has biological and clinical implications. J Pathol. 215:21–30. 2008.

11. Dho YS, Jung KW, Ha J, Seo Y, Park CK, Won YJ, et al. An updated nationwide epidemiology of primary brain tumors in Republic of Korea, 2013. Brain Tumor Res Treat. 5:16–23. 2017.

12. Fujimoto T, Miyayama Y, Fuyuta M. The origin, migration and fine morphology of human primordial germ cells. Anat Rec. 188:315–330. 1977.

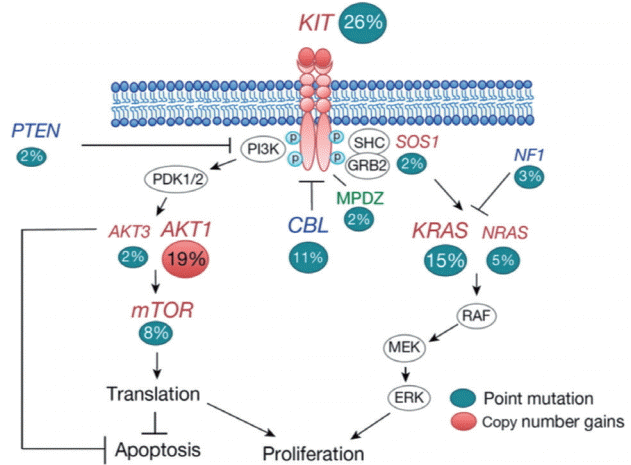
13. Fukushima S, Otsuka A, Suzuki T, Yanagisawa T, Mishima K, Mukasa A, et al. Mutually exclusive mutations of KIT and RAS are associated with KIT mRNA expression and chromosomal instability in primary intracranial pure germinomas. Acta Neuropathol. 127:911–925. 2014.

14. Fukushima S, Yamashita S, Kobayashi H, Takami H, Fukuoka K, Nakamura T, et al. Genome-wide methylation profiles in primary intracranial germ cell tumors indicate a primordial germ cell origin for germinomas. Acta Neuropathol. 133:445–462. 2017.

15. Gashaw I, Dushaj O, Behr R, Biermann K, Brehm R, Rübben H, et al. Novel germ cell markers characterize testicular seminoma and fetal testis. Mol Hum Reprod. 13:721–727. 2007.

16. Gillis AJ, Stoop H, Biermann K, van Gurp RJ, Swartzman E, Cribbes S, et al. Expression and interdependencies of pluripotency factors LIN28, OCT3/4, NANOG and SOX2 in human testicular germ cells and tumours of the testis. Int J Androl. 34(4 Pt 2):e160–e174. 2011.

17. Goddard NC, McIntyre A, Summersgill B, van Gurp RJ, Gilbert D, Kitazawa S, Shipley J. KIT and RAS signalling pathways in testicular germ cell tumours: new data and a review of the literature. Int J Androl. 30:337–348; discussion 349. 2007.

18. Goodwin TL, Sainani K, Fisher PG. Incidence patterns of central nervous system germ cell tumors: a SEER study. J Pediatr Hematol Oncol. 31:541–544. 2009.
19. Høyer PE, Byskov AG, Møllgård K. Stem cell factor and c-Kit in human primordial germ cells and fetal ovaries. Mol Cell Endocrinol. 234:1–10. 2005.

20. Ichimura K, Fukushima S, Totoki Y, Matsushita Y, Otsuka A, Tomiyama A, et al. Recurrent neomorphic mutations of MTOR in central nervous system and testicular germ cell tumors may be targeted for therapy. Acta Neuropathol. 131:889–901. 2016.

21. Ishida M, Moore GE. The role of imprinted genes in humans. Mol Aspects Med. 34:826–840. 2013.

22. Kamakura Y, Hasegawa M, Minamoto T, Yamashita J, Fujisawa H. C-kit gene mutation: common and widely distributed in intracranial germinomas. J Neurosurg. 104(3 Suppl):173–180. 2006.

23. Kato T, Sawamura Y, Tada M, Murata J, Abe H, Shirato H, et al. Occult neurohypophyseal germinomas in patients presenting with central diabetes insipidus. Neurosurg Focus. 5:e6. 1998.

24. Kellie SJ, Boyce H, Dunkel IJ, Diez B, Rosenblum M, Brualdi L, et al. Intensive cisplatin and cyclophosphamide-based chemotherapy without radiotherapy for intracranial germinomas: failure of a primary chemotherapy approach. Pediatr Blood Cancer. 43:126–133. 2004.

25. Kim CY, Choi JW, Lee JY, Kim SK, Wang KC, Park SH, et al. Intracranial growing teratoma syndrome: clinical characteristics and treatment strategy. J Neurooncol. 101:109–115. 2011.

26. Kim JW, Kim WC, Cho JH, Kim DS, Shim KW, Lyu CJ, et al. A multimodal approach including craniospinal irradiation improves the treatment outcome of high-risk intracranial nongerminomatous germ cell tumors. Int J Radiat Oncol Biol Phys. 84:625–631. 2012.

27. Lai IC, Wong TT, Shiau CY, Hu YW, Ho DM, Chang KP, et al. Treatment results and prognostic factors for intracranial nongerminomatous germ cell tumors: single institute experience. Childs Nerv Syst. 31:683–691. 2015.

28. Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 86:446–455. 1997.

29. McCarthy BJ, Shibui S, Kayama T, Miyaoka E, Narita Y, Murakami M, et al. Primary CNS germ cell tumors in Japan and the United States: an analysis of 4 tumor registries. Neuro Oncol. 14:1194–1200. 2012.

30. Mootha SL, Barkovich AJ, Grumbach MM, Edwards MS, Gitelman SE, Kaplan SL, et al. Idiopathic hypothalamic diabetes insipidus, pituitary stalk thickening, and the occult intracranial germinoma in children and adolescents. J Clin Endocrinol Metab. 82:1362–1367. 1997.

31. Murray MJ, Bartels U, Nishikawa R, Fangusaro J, Matsutani M, Nicholson JC. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 16:e470–e477. 2015.

32. Murray MJ, Horan G, Lowis S, Nicholson JC. Highlights from the Third International Central Nervous System Germ Cell Tumour symposium: laying the foundations for future consensus. Ecancermedicalscience. 7:333. 2013.

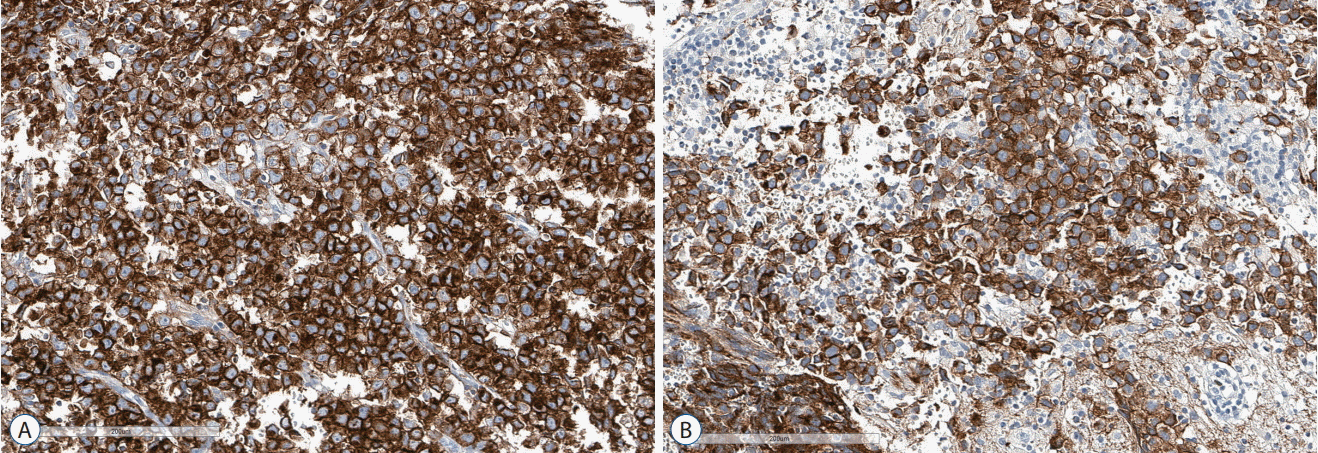
33. Nakamura H, Takeshima H, Makino K, Kuratsu J. C-kit expression in germinoma: an immunohistochemistry-based study. J Neurooncol. 75:163–167. 2005.

34. Netto GJ, Nakai Y, Nakayama M, Jadallah S, Toubaji A, Nonomura N, et al. Global DNA hypomethylation in intratubular germ cell neoplasia and seminoma, but not in nonseminomatous male germ cell tumors. Mod Pathol. 21:1337–1344. 2008.

35. Odagiri K, Omura M, Hata M, Aida N, Niwa T, Ogino I, et al. Treatment outcomes, growth height, and neuroendocrine functions in patients with intracranial germ cell tumors treated with chemoradiation therapy. Int J Radiat Oncol Biol Phys. 84:632–638. 2012.

36. Ogiwara H, Kiyotani C, Terashima K, Morota N. Second-look surgery for intracranial germ cell tumors. Neurosurgery. 76:658–661; discussion 661-662. 2015.

37. Okada Y, Nishikawa R, Matsutani M, Louis DN. Hypomethylated X chromosome gain and rare isochromosome 12p in diverse intracranial germ cell tumors. J Neuropathol Exp Neurol. 61:531–538. 2002.

38. Oosterhuis JW, Stoop H, Honecker F, Looijenga LH. Why human extragonadal germ cell tumours occur in the midline of the body: old concepts, new perspectives. Int J Androl. 30:256–263; discussion 263-264. 2007.

39. Padilla BE, Vu L, Lee H, MacKenzie T, Bratton B, O’Day M, et al. Sacrococcygeal teratoma: late recurrence warrants long-term surveillance. Pediatr Surg Int. 33:1189–1194. 2017.

40. Phi JH, Cho BK, Kim SK, Paeng JC, Kim IO, Kim IH, et al. Germinomas in the basal ganglia: magnetic resonance imaging classification and the prognosis. J Neurooncol. 99:227–236. 2010.

41. Phi JH, Kim SK, Lee J, Park CK, Kim IH, Ahn HS, et al. The enigma of bifocal germ cell tumors in the suprasellar and pineal regions: synchronous lesions or metastasis? J Neurosurg Pediatr. 11:107–114. 2013.

42. Phi JH, Kim SK, Lee YA, Shin CH, Cheon JE, Kim IO, et al. Latency of intracranial germ cell tumors and diagnosis delay. Childs Nerv Syst. 29:1871–1881. 2013.

43. Phi JH, Kim SK, Park SH, Hong SH, Wang KC, Cho BK. Immature teratomas of the central nervous system: is adjuvant therapy mandatory? J Neurosurg. 103(6 Suppl):524–530. 2005.

44. Reuter VE. Origins and molecular biology of testicular germ cell tumors. Mod Pathol. 18 Suppl 2:S51–S60. 2005.

45. Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol. 11:37–49. 2010.

46. Rogers SJ, Mosleh-Shirazi MA, Saran FH. Radiotherapy of localised intracranial germinoma: time to sever historical ties? Lancet Oncol. 6:509–519. 2005.

47. Sakuma Y, Sakurai S, Oguni S, Satoh M, Hironaka M, Saito K. c-kit gene mutations in intracranial germinomas. Cancer Sci. 95:716–720. 2004.

48. Sano K. Pathogenesis of intracranial germ cell tumors reconsidered. J Neurosurg. 90:258–264. 1999.

49. Sawamura Y, Ikeda J, Shirato H, Tada M, Abe H. Germ cell tumours of the central nervous system: treatment consideration based on 111 cases and their long-term clinical outcomes. Eur J Cancer. 34:104–110. 1998.

50. Sette C, Dolci S, Geremia R, Rossi P. The role of stem cell factor and of alternative c-kit gene products in the establishment, maintenance and function of germ cells. Int J Dev Biol. 44:599–608. 2000.
51. Sheikine Y, Genega E, Melamed J, Lee P, Reuter VE, Ye H. Molecular genetics of testicular germ cell tumors. Am J Cancer Res. 2:153–167. 2012.
52. Sukov WR, Cheville JC, Giannini C, Carlson AW, Shearer BM, Sinnwell JP, et al. Isochromosome 12p and polysomy 12 in primary central nervous system germ cell tumors: frequency and association with clinicopathologic features. Hum Pathol. 41:232–238. 2010.

53. Tanabe M, Mizushima M, Anno Y, Kondou S, Dejima S, Hirao DJ, et al. Intracranial germinoma with Down’s syndrome: a case report and review of the literature. Surg Neurol. 47:28–31. 1997.

54. Teilum G, Albrechtsen R, Norgaard-Pedersen B. The histogeneticembryologic basis for reappearance of alpha-fetoprotein in endodermal sinus tumors (yolk sac tumors) and teratomas. Acta Pathol Microbiol Scand A. 83:80–86. 1975.

55. Tian Q, Frierson HF Jr, Krystal GW, Moskaluk CA. Activating c-kit gene mutations in human germ cell tumors. Am J Pathol. 154:1643–1647. 1999.

56. Trama A, Berrino F. The epidemiology of malignant germ cell tumors: the EUROCARE study in Nogales FF, Jimenez RE (eds) : Pathology and Biology of Human Germ Cell Tumors. Germany: Springer-Verlag GmbH;2017. p. 11–21.
57. Wang L, Yamaguchi S, Burstein MD, Terashima K, Chang K, Ng HK, et al. Novel somatic and germline mutations in intracranial germ cell tumours. Nature. 511:241–245. 2014.

58. Weiner HL, Lichtenbaum RA, Wisoff JH, Snow RB, Souweidane MM, Bruce JN, et al. Delayed surgical resection of central nervous system germ cell tumors. Neurosurgery. 50:727–733; discussion 733-734. 2002.

59. Wolf SS, Patchev VK, Obendorf M. A novel variant of the putative demethylase gene, s-JMJD1C, is a coactivator of the AR. Arch Biochem Biophys. 460:56–66. 2007.

60. Yoshida M, Matsuoka K, Nakazawa A, Yoshida M, Inoue T, Kishimoto H, et al. Sacrococcygeal yolk sac tumor developing after teratoma: a clinicopathological study of pediatric sacrococcygeal germ cell tumors and a proposal of the pathogenesis of sacrococcygeal yolk sac tumors. J Pediatr Surg. 48:776–781. 2013.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download