INTRODUCTION
In addition to clinical symptoms and signs, radiologic and laboratory investigation is commonly used to support the diagnosis and assessment of an inflammatory process [
7]. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are useful for screening and assessment in the diagnosis and treatment of infectious spondylitis. Compared to the ESR, CRP is a more sensitive and specific acute phase reactant and is more responsive to changes in the course of infection [
6,
13]. However, an elevation in CRP and/or ESR should not be taken as pathognomonic for infection [
7] .
Conservative therapy with appropriate antibiotics is essential for most patients with infectious spondylitis [
13]. With resolution of infectious spondylitis, the CRP and ESR levels should decrease [
6,
7]. If these inflammatory markers remain consistently elevated despite use of intravenous (IV) antibiotics, a repeat radiological study should be considered to look for any infectious lesions that may require a longer course of IV therapy [
5,
13]. However, side effects can occur with any class of drugs and adverse reactions of antibiotics can range from mild allergic reactions to serious and fulminant adverse events. These side effects are also extremely variable from patient to patient and from antibiotic to antibiotic [
17]. A side effect of antibiotics is a paradoxical increase in inflammatory marker levels despite cure of infection. The author presents two cases of antibiotic-induced increase in inflammatory markers in cured infectious spondylitis. The patients were successfully treated after stopping the antibiotic therapy.
Go to :

DISCUSSION
Infectious spondylitis is defined as an infection of one or more components of the spine by a specific organism [
7]. Most patients with infective spondylitis can be treated conservatively with antibiotics [
5,
6]. Because the clinical symptoms vary widely in the early stages, it is difficult to differentiate infectious spondylitis from other diseases, resulting in delayed diagnosis or misdiagnosis. The onset of symptoms is commonly insidious, with spinal pain being the most common presenting complaint. Although more than 90% of cases are pyogenic [
6,
9], fever is typically not present and occurs in less than 20% of patients [
6,
13].
The WBC count, ESR, and CRP are indicators of inflammation in patients with infectious spondylitis. The WBC count is elevated in 40–66% of patients, and is not particularly useful in making a diagnosis of infectious spondylitis [
13]. The ESR is a sensitive indicator of infectious spondylitis, and is positive in 76–81% of patients at the time of diagnosis, with levels ranging from 43–87 mm/h in pyogenic spondylitis [
4,
13]. Elevation of ESR is correlated with the inflammatory response but is not specific for infection. The ESR is often significantly affected by many factors other than the acute phase reaction [
7] , including the plasma albumin level; the size, shape, and number of red blood cells; and non-acute phase reaction proteins, in particular normal and abnormal immunoglobulins. The lack of specificity of the ESR means the test is more likely to be falsely positive than the CRP. Moreover, the slow response of ESR to the acute phase reaction leads to false negatives early in an inflammatory process [
8]. Despite the nonspecific nature of an elevation in the ESR, this test provides additional data regarding the possible presence of infection and some information on the response to treatment. CRP is a useful marker of the acute phase reaction as it responds quickly to the inflammatory process [
7,
11]. It is an acute phase protein synthesized by hepatocytes. Although CRP activation of complement increases inflammation and tissue damage, it also has some anti-inflammatory activity, and acts as a promoter and down-regulator of inflammation [
11]. CRP is elevated in 90% or more of patients with spinal infection, but is more specific than ESR and normalizes postoperatively or after appropriate treatment of an infectious process, more rapidly than the ESR [
7,
11]. A majority of patients with infective spondylitis can be treated without surgery. Although the optimal duration is not well defined, several studies recommend 6--8 weeks of IV antibiotics and others recommend only 4 weeks [
5,
6,
13]. Insufficient IV antibiotic therapy for less than 4 weeks may result in a high recurrence rate. Some authors advise use of IV antibiotics until the CRP is norma [l6].
Antibiotics are used for the treatment and prevention of many infectious disorders and are thought to be safe when applied properly. However, like all drugs, they also show various adverse effects in some patient conditions [
17]. Antibiotic complications can affect the hematologic, cardiac, respiratory, gastrointestinal, hepatobiliary, renal, genitourinary, rheumatologic, dermatologic, and neurologic systems, and can cause a drug fever [
17]. Multiple antibiotics are associated with QT prolongation and may increase the risk of sudden cardiac arrest due to torsades de pointes [
10]. Patients treated with antibiotics frequently experience diarrhea. Although
Clostridium difficile infection is obviously of great concern, the majority of diarrhea cases will not be attributable to this infection. Nausea is frequently encountered and it is often difficult to identify a specific cause, although it should be noted that there are some antibiotics for which this is a very common side effect [
3]. The most common dermatologic adverse reaction associated with antibiotic therapy is a drug-induced exanthem, or “drug rash.” Parenteral therapy with beta-lactams (penicillins and cephalosporins) is associated with drug-induced fever, as is therapy with several other antibiotics including sulfonamides [
15].
A research carried out at six pharmacovigilance centers in Korea reported that antibiotics including vancomycin were the most frequent causes of adverse reactions of drug, and that cutaneous symptoms were the most common manifestations in adverse reactions [
12]. In this study, 3.1% of 1418 cases were associated with vancomycin. An et at. [
1]. reported that the skin rashes associated with increased peripheral eosinophil, representing suspected immune-mediated delayed hypersensitivity reactions, are a common adverse reaction of vancomycin. Eosinophilia is considered when absolute eosinophil count exceeds 500/µL in peripheral blood. Peripheral eosinophilia can be caused by parasitic infections, allergy, drug reactions, leukemia, and non-hematologic malignancies [
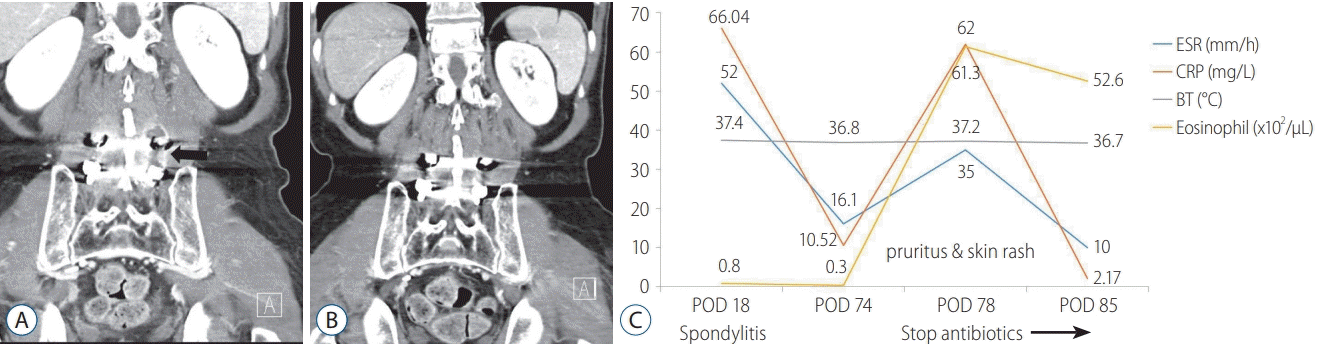
14]. The author treated infectious spondylitis with vancomycin in case 1 patient. During the period of treatment with vancomycin, inflammatory markers decreased gradually. However, after 2 months, the inflammatory markers subsequently increased rapidly, and whole-body pruritus and skin rash were observed Laboratory tests revealed significant eosinophilia. The author suspected that an adverse effect of allergic reaction of adverse drug effects.
Case 2 patient treated with moxifloxacin and cefixime had no eosinophilia or skin symptoms other than abdominal pain due to diarrhea. Antibiotic-associated diarrhea is defined as unexplained diarrhea association with antibiotic administration [
3]. Although the frequency of antibiotics-associated diarrhea depends on the antibiotics, it occurs in approximately 5.2 to 6.2% of patients who are treated with moxifloxacin and 15 to 20% of those who receive cefixime [
2,
3]. A common first step is to identify cases of antibiotic-associated diarrhea that are due to
Clostridium difficile infection, because this is the most common identifiable and treatable pathogen. The tests used for diagnosis depend on the type of laboratory investigations available. Enzyme immunoassays for detecting toxin A or toxins A and B are generally available [
3]. In case 2,
Clostridium difficile toxin was not detected. Only 10–20% of the stool specimens submitted for testing of
Clostridium difficile toxins are reported as positive [
16]. Antibiotic-associated diarrhea can also be caused by other enteric pathogens, direct effects of antibiotics on the intestinal mucosa, and the metabolic consequences of reduced concentrations of fecal flora. Many patients with enteric disease caused by antibiotics have a response to withdrawal of the inducing agent [
3,
16]. The author thought that a direct effect of antibiotics affected the digestive system has increased levels of inflammatory marker in case 2 patient. After stopping antibiotics, the inflammatory markers in both cases rapidly returned to normal.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download