INTRODUCTION
Cubital tunnel syndrome (CuTS) caused by compression on the ulnar nerve is the second most common compression neuropathy that occurs in the upper extremity, after carpal tunnel syndrome [
3]. Although most cases of CuTS are idiopathic, it is known to be associated with elbow trauma, cubitus valgus or varus, arthritis, ulnar nerve instability, tumor, metabolic or systemic disease, hypertrophic medial head of the triceps, and anconeus epitrochlearis (AE) muscle [
1,
14,
15,
20,
21].
AE muscle, which originates from the medial humeral epicondyle and attaches to the olecranon, protects the ulnar nerve and prevents subluxation, but it can also be viewed as a potential cause of ulnar nerve compression. The literature describes the AE muscle as one of the causes of CuTS, but its prevalence in actual clinical settings is very rare [
8-
10,
18]. The objective of the present study was to report the incidene, clinical features and surgical outcomes of 12 patients with AE muscle who underwent surgery for CuTS.
Go to :

MATERIALS AND METHODS
This retrospective case series was initiated following institutional review board (IRB) approval of the Catholic university of Korea (IRB No. PC17RESI0025). The present study initially reviewed 142 patients who underwent surgery for CuTS from November 2007 to October 2015. Among 142 patients who underwent surgery, AE muscle was discovered in 12 patients; these patients were assigned to the AE group and the remaining 130 patients were assigned to the other group. The two groups were compared in terms of age, gender, dominant hand, symptom duration (duration from onset of symptoms to surgery), and hand weakness. The surgery used a medial retroepicondylar approach to excise the AE muscle or ligament, and released all compressive structures around the ulnar nerve. The ulnar nerve was transposed to a position anterior to the medial epicondyle and placed superficial to the f lexor-pronator muscle group but deep to its fascia which wraps the ulnar nerve (subfascial anterior transposition of the ulnar nerve). The patients were allowed joint movement without any restriction starting at 2 weeks postoperative, and improvement in symptoms was evaluated during the final follow-up using disability of arm shoulder and hand (DASH) and visual analogue scale (VAS) scores. The DASH is a 30-item self-report questionnaire designed to evaluate musculoskeletal disorders of the upper limbs and measure symptoms and function of the patients.
Statistical analysis was performed using SPSS version 20.0 statistical software for Windows (IBM Corp., Armonk, NY, USA). Comparative analysis of age and symptom duration between the AE and the other group was performed using Mann-Whitney U test, while comparative analysis of gender, dominant hand, and hand weakness was performed using Fisher’s extract test. For the analyses, p-values <0.05 were considered statistically significant.
Go to :

RESULTS
Among the 142 patients who underwent surgery for CuTS, AE muscle was observed in 12 patients (8.5%). The AE group (
Table 1) included nine males and three females, with a mean age of 42.7 years (range, 23–64 years). The average follow-up period for the AE group was 20.3 months (range, 12–36 months). Electromyography was performed on all patients and magnetic resonance imaging (MRI) was performed in eight patients with palpable mass on the medial side of the elbow. Preoperatively none of the patients showed ulnar nerve instability. According to Dellon’s criteria, three patients were classified as mild degree, seven as moderate degree, and two as severe degree. The mean age of the patients was 42.7 and 52.6 years in the AE and the other groups, respectively; the AE group was significantly younger (
p=0.02). There was no significant difference in gender between groups. The percentage of patients with the dominant hand being affected was 83.3% (10/12) in the AE group and 53% (69/130) in the other group, representing significant difference (
p=0.041). The AE group had a significantly shorter symptom duration than the other group (5 vs. 13.2 months). The two groups did not show statistically significant difference in preoperative hand weakness (
p=0.42) (
Table 2). Mass was palpated by 8 out of 12 patients, although there was difference in size on MRI that was performed in this patients, confirmation was possible. Among patients in whom AE muscle was found, 11 cases of AE muscle and one case of AE ligament were seen intraoperatively. In all 12 cases, relatively distinct findings of ulnar nerve compression were suspected from the surgical field of view, but actual nerve abnormalities such as nerve indentation and pseudo-tumor formation were found in only three cases. This suggests that CuTS caused by AE muscle is a dynamic compressive neuropathy. At final follow-up, DASH and VAS scores were significantly improved (
Table 3).
Table 1.
Demographic data of the anconeus epitrochlearis group
|
Pt |
Sex/age (years) |
Occupation |
Affected/dominant |
Sx. duration (months) |
Ulnar N instability |
Dellon stage |
F/U period (months) |
|
1 |
M/23 |
Health trainer |
Rt/Rt |
12 |
None |
Mild |
18 |
|
2 |
M/26 |
Judo |
Lt/Rt |
2 |
None |
Mild |
16 |
|
3 |
F/35 |
Tennis player |
Rt/Rt |
4 |
None |
Mod |
22 |
|
4 |
M/37 |
Officer |
Lt/Lt |
1 |
None |
Mod |
24 |
|
5 |
M/64 |
Heavy labor |
Lt/Lt |
10 |
None |
Severe |
36 |
|
6 |
M/55 |
Carpenter |
Rt/Rt |
2 |
None |
Mod |
24 |
|
7 |
F/56 |
Restaurant |
Rt/Rt |
4 |
None |
Mod |
16 |
|
8 |
F/42 |
Housewife |
Rt/Rt |
3 |
None |
Mod |
16 |
|
9 |
M/58 |
Farmer |
Rt/Rt |
8 |
None |
Mod |
12 |
|
10 |
M/46 |
Electrician |
Rt/Rt |
6 |
None |
Severe |
24 |
|
11 |
M/32 |
Soldier |
Rt/Rt |
2 |
None |
Mod |
18 |
|
12 |
M/38 |
Officer |
Lt/Rt |
6 |
None |
Mild |
18 |

Table 2.
Clinical differences between the anconeus epitrochlearis group and the other group
|
AE group |
The other group |
p-value |
|
Age (years) |
42.7±13.9 |
52.6±12.6 |
0.02*
|
|
Sex (male : female) |
9 : 3 |
84 : 46 |
0.52 |
|
Dominant hand |
10 (83.3) |
69 (53) |
0.041*
|
|
Symptom duration |
5±3.9 |
13.2±12.2 |
0.011*
|
|
Hand weakness |
9 (75) |
88 (68) |
0.42 |

Table 3.
The preoperative and postoperative status of the anconeus epitrochlearis group
|
Preoperative |
Postoperative |
p-value |
|
DASH |
36.2±16.2 |
19.4±14.3 |
<0.05 |
|
VAS |
5.8±1.4 |
2.3±1.8 |
<0.05 |

Case presentations
Case 1 (patient No. 1)
A 23-year-old male patient was admitted for chief complaints of muscle weakness and numbness in the right ring and little finger, which began a year earlier and became worse 3 months prior to presentation. The patient’s occupation was a health trainer and he complained that the symptoms became more aggravated when force was applied to the arms during exercise or the elbow was bent for a prolonged period.
Physical examination found medial elbow pain, blunted sensation in the ulnar nerve area of the hand, and weakened ability to flex the 4th and 5th fingers. The elbow flexion test showed positive findings. Since CuTS was suspected based on the physical examination results, electromyography was performed, but the results showed normal findings. Simple radiographs did not show any specific findings (
Fig. 1A and
B). MRI showed an atypical oval-shaped muscle extending from the medial epicondyle to the medial side of the olecranon (
Fig. 1C and
D). Intraoperatively, the AE muscle, 2.0×1.5 cm in size and extending from the olecranon to the medial epicondyle, was excised (
Fig. 1E and
F); atrophy of the compressed area and a hypertrophic ulnar nerve in the proximal area were then observed (
Fig. 1G). After excision of the AE muscle, subfascial anterior transposition of the ulnar nerve was performed (
Fig. 1H). Splint was applied for 2 weeks postoperatively, and at 1 year postoperative, all symptoms were resolved (preoperative DASH : 35, VAS : 7; postoperative DASH : 16, VAS : 2).
 | Fig. 1.A and B : A 23-year-old male patient had surgery for cubital tunnel syndrome. The radiograph showed no definitive abnormality. C and D : Transverse and sagittal T2-weighted magnetic resonance imaging of the elbow showed anconeus epitrochlearis (AE) muscle (asterisk). E : Intraoperative finding revealed AE muscle (asterisk) above the cubital tunnel. F : After resection of the AE muscle, the compressed ulnar nerve was shown (G). H : Subfascial anterior transposition of the ulnar nerve was performed. ME : medial epicondyle, Ole : olecranon, Ulnar N : ulnar nerve. 
|
Go to :

DISCUSSION
The AE muscle is a congenital accessory muscle that originates from the medial humeral epicondyle and attaches to the medial side of olecranon. It is rare in humans [
5,
16], and cadaveric studies have reported varying frequency of 4–34% in normal people [
4,
16]. However, the prevalence of CuTS caused by this muscle in the actual clinical settings and its clinical significance are poorly understood. With respect to the differences in actual percentages of AE muscle being present and manifesting clinical symptoms, Hirasawa et al. [
12] surmised that this muscle covers the back of the ulnar nerve and acts as a potential factor that further compresses the ulnar nerve during elbow flexion, and its etiology is muscle hypertrophy due to lifting heavy objects or intense exercise, especially by athletes. In other words, clinical symptoms do not appear in everyone who has such congenital accessory muscle, with symptoms of ulnar nerve compression appearing only in certain individuals with developed muscles. Wilson et al. [
24] reported that AE muscle may be protective against the development of CuTS, and the mechanism of protection may be that this muscle decreases the rigidity of the entrance into the cubital tunnel. However, when an AE muscle does contribute to CuTS, it is likely secondary to hypertrophy of the muscle from repetitive use. Even the present study was unable to accurately identify any association between the onset of CuTS caused by AE muscle and the patient’s occupation and activity level. However, in looking at the occupation and activity level of the 12 patients in whom AE muscle was found, it is believed that most of them used their upper extremity significantly. Compared to idiopathic CuTS, CuTS caused by AE muscle is reported to occur more often in younger patients and in males, and disease progression is more rapid [
2,
4,
12,
18,
22]. In the present study, the AE group was younger than the other group and the disease was more prevalent in the dominant hand, while symptom duration was significantly shorter in the AE group than in the other group. Compared to the other group, there was no significant difference based on gender, but in the AE group, there were more males (9/12, 75%) than females.
In the present study, patients with CuTS caused by AE muscle had intermittent symptoms of ulnar nerve compression, showing dynamic compression of the ulnar nerve that was mild during rest and more severe during exercise or when the elbow was bent for a prolonged time. The evidences of dynamic compression of the ulnar nerve were as follows. First, differences in severity did exist, but the fact that two patients showed normal electromyographic findings despite all cases having distinct symptoms of ulnar neuropathy supports the above claim. Second, in all 12 cases, relatively distinct findings of ulnar nerve compression were suspected from the surgical field of view, but actual nerve abnormalities such as nerve indentation and pseudo-tumor formation were found in only three cases. We believe that in such cases, measuring grip/pinch strength or measuring nerve conduction velocity in the elbow area during rest and after exercise may be helpful in making the diagnosis [
10,
17]. Byun et al. [
4] discussed the findings of electromyography (EMG) in patients with ulnar neuropathy caused by AE muscle compared with those with idiopathic CuTS. They suggested that an AE muscle should be considered a possible cause in young male patients with short symptom duration, and a short segmental ulnar motor conduction study may detect AE muscle induced neuropathy. They reported that ulnar nerve motor conduction studies showed conduction block, which is defined as a reduction of area/amplitude of at least 50% at proximal vs distal site of stimulation, in AE muscle induced ulnar neuropathy. Another comparative study with idiopathic CuTS, velocity drop of the ulnar nerve was significantly associated with the presence of an AE muscle [
18]. In our study, the sensory nerve conduction study was normal on the EMG of the AE group, but the motor nerve conduction velocities between the proximal 2 cm and distal 2 cm of medial epicondyle decreased. This velocity drop suggests a characteristic of CuTS by an AE muscle with subacute onset of symptoms rather than the chronic demyelinating process that is seen in idiopathic ulnar neuropathy.
In the present study, 8 of 12 patients had palpable mass in the cubital tunnel before surgery. If activity-related symptoms and a palpable mass in the elbow joint area are present, recognizing the possibility of CuTS due to the AE muscle combined with performance of MRI might be helpful for the diagnosis. AE muscle showed varying size and shape, and in one particular case (patient No. 5,
Fig. 2), intraoperative findings showed a ligamentous structure with the same points of origin and insertion, as well as the same direction, as the AE muscle; this was believed to be an AE ligament, as reported by Tiong and Kelly [
22]. O’Driscoll et al. [
19] reported that a ligamentous structure oriented in the same direction as the AE muscle was muscle that degenerated into ligament, and termed this AE ligament. However, CuTS caused by such AE ligament is known to be extremely rare. Although ulnar nerve compression may be caused by hypertrophy of the AE muscle or edema, it is believed that a tight AE ligament (thick fibrous tissue) may also be a potential cause of ulnar nerve compression.
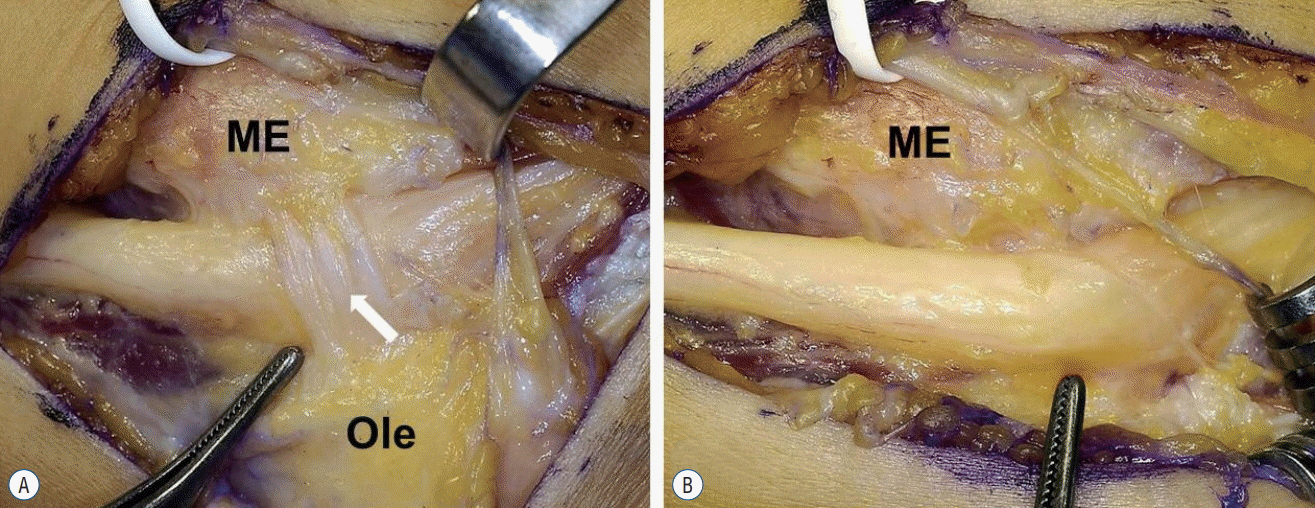 | Fig. 2.Intra-operative findings. A : An anconeus epitroclearis (AE) ligament (arrow). B : After resection of AE ligament. ME : medial epicondyle, Ole : olecranon. 
|
Surgical treatment for CuTS can be classified largely into neurolysis, simple decompression [
7], medial epicondylectomy, and anterior transposition; anterior transposition can be divided into subcutaneous, submuscular, and subfascial transposition, depending on the location. The same surgical methods may be applied to CuTS caused by AE muscle as well. Masear et al. [
16] and Gervasio and Zaccone [
11] reported improvement in symptoms with excision of only the muscle and simple decompression, while Hodgkinson and McLean [
13] reported favorable outcomes with muscle excision and medial epicondylectomy. With respect to which method is the ideal surgical treatment, various techniques are being employed by each surgeon since no objective standards are available. Many surgical treatments are recommended for the CuTS caused by AE muscle, and the most frequent is excision of the AE muscle and simple decompression of the ulnar nerve [
11,
16,
20]. Complete excision of the muscle and simple decompression of the nerve are widely accepted as definitive treatment, but whether to transpose the ulnar nerve remains controversial. O’Hara and Stone [
20] reported that if isolated compression can be treated effectively by decompression alone at the site of the anomalous muscle, there is no need for wider decompression or transposition of the ulnar nerve. However, excellent results have been reported by Chalmers [
6] that it was wiser to perform full exploration, decompression, and anterior transposition of the ulnar nerve because more common sources of compression may coexist. Erdem Bagatur et al. [
9] also performed anterior transposition of the ulnar nerve to prevent nerve dislocation because extensive neurolysis was needed. We performed not only excision of AE muscle but also released of all potential compressive structures. And then we followed by anterior transposition to prevent ulnar nerve instability and eventually to reduce the likelihood of recurrence [
23].
The limitations in the present study included the fact that it was retrospective in design, had a small number of cases, which limits the ability to generalize the characteristics associated with AE muscle, and had a short follow-up period. And differences in surgical methods used could not be determined since we performed anterior transposition of the ulnar nerve on all patients suspected of having CuTS caused by AE muscle. A larger sample size and longer follow-up period are needed to address these issues, while it is also necessary to evaluate treatment methods to determine the most appropriate intervention for this disease. Finally, we also believe that objective and quantitative comparative studies are needed to determine the pathophysiological differences between patients with CuTS caused by AE muscle versus CuTS due to other causes.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download