DISCUSSION
In the past few decades, the incidence of TBI has declined among young adults, potentially because of greater public awareness and improved preventative measures; in contrast, elderly patients continue to experience the highest and fastest growing TBI rates compared with patients in other age groups [
3,
5,
6]. In general, age is closely associated with increased poor outcomes and mortality after TBI [
7,
13,
15]. Outcomes and poor prognostic factors in geriatric TBI are increased age, low GCS score, pupillary dilatation, intracranial mass lesion, motor vehicle accidents, falls, jumps, intracranial pressure ≥20 mmHg, early hypoxia, associated systemic injury, and systemic complications [
7,
12,
13,
15,
17]. In addition, elderly patients are known to experience worse outcomes and require prolonged recovery compared with patients in other age groups even after controlling for injury severity [
6].
Table 1 shows that the demographic clinical characteristics of elderly patients with TBI, as stratified by sex. As the age increased, a decreasing trend was observed in the number of cases with TBI in 904 patients because of the decrease in the elderly population. This trend was more prominent in men. However, the incidence of TBI in female patients is the highest at an age of 70–79 years; this difference according to sex was statistically significant, possibly because of increasing women’s social activities and the average life expectancy of women being longer than that of men. No significant differences were observed in demographic clinical characteristics between male and female patients with TBI, except for the age distribution. Falls are the leading cause of TBI in older adults, and TA are second in acute care aligns with results from around the world [
3,
16]. Our study also revealed falls as the most common cause of TBI, followed by TA. However, elderly patients in Korea had higher ratios of TA than did those in other countries [
16]. The incidence of falls was higher in the female group (46.4%) and of TAs was higher in men (28.3%) in this study. Fu et al. [
6] reported that patients with a fall-related TBI tended to be women, older, and have a higher comorbidity than those with a non-fall related TBI. Chan et al. [
3] insisted that the marked increase in fall-related TBI illustrates the need for an increased focus on fall prevention strategies among older adults.
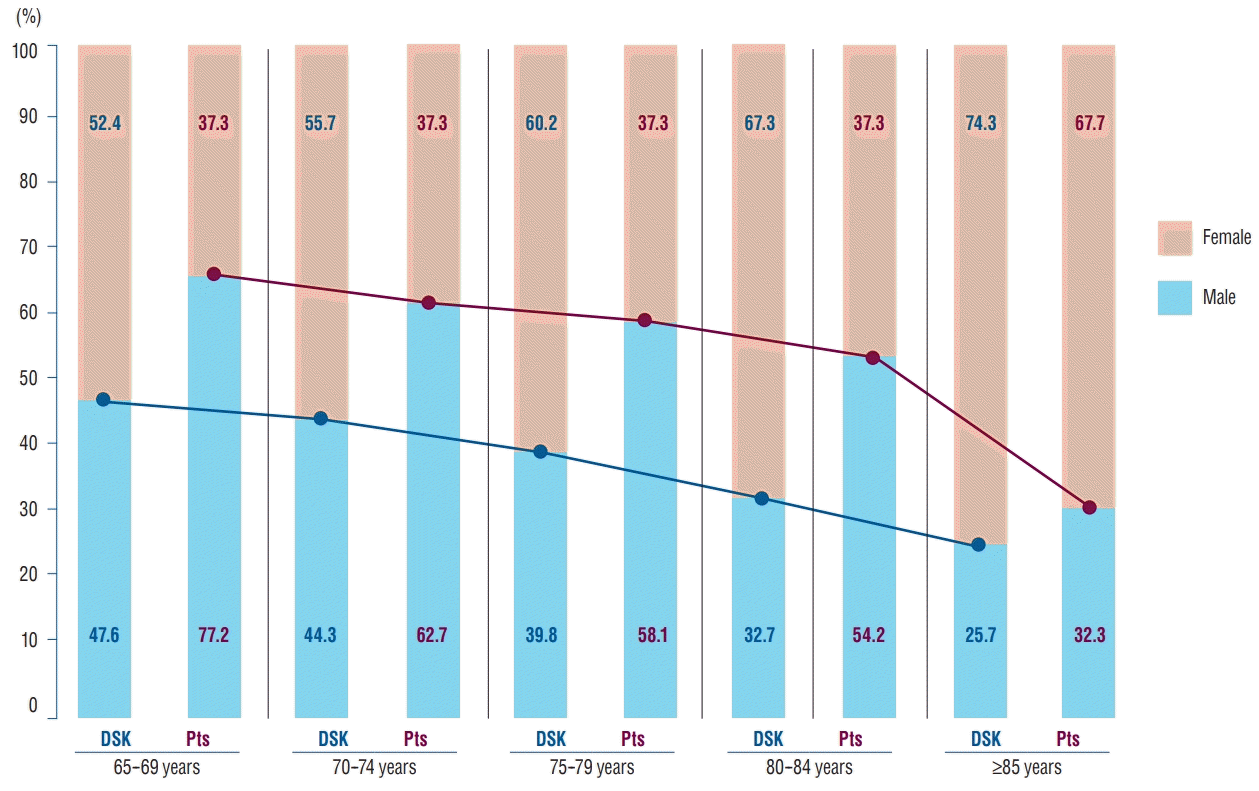
Irrespective of age, the incidence of TBI has been reported to be higher in men in the literature [
7,
11-
14]. As shown in
Table 2 and
Fig. 1, men experience about twice as many TBI events as do women in all age groups. Despite the increasing life expectancy and social activities of elderly women, the incidence according to the age group is roughly similar and not significantly different based on the demographic statistics of the KNSO in March 2014 [
9].
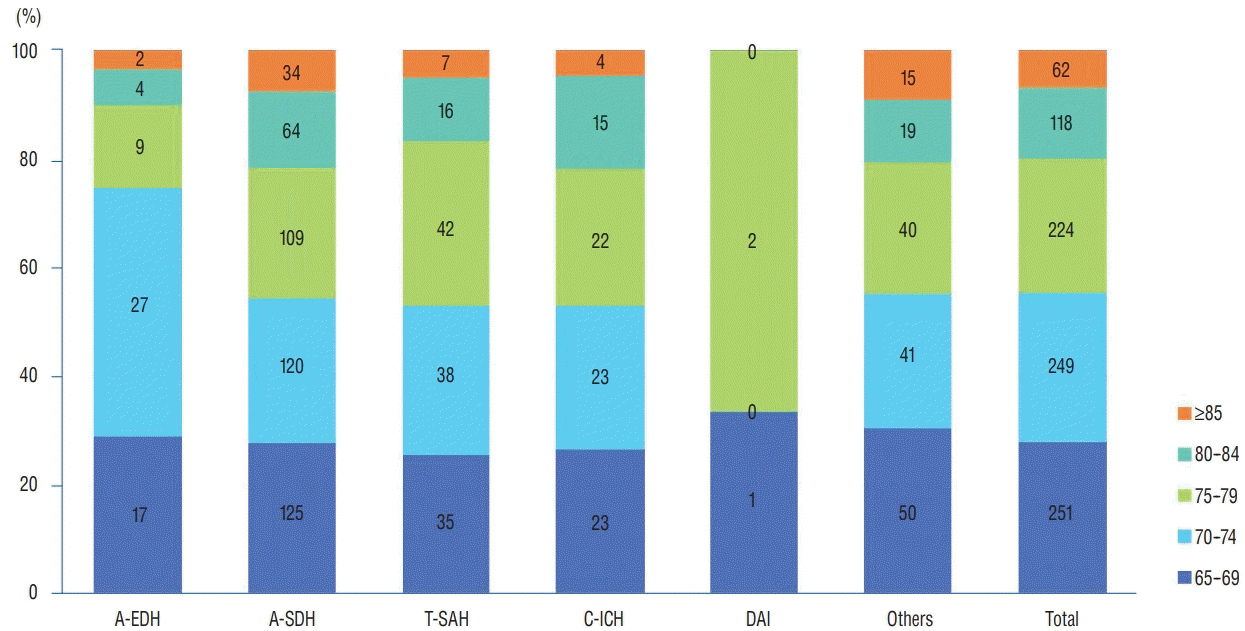
In this study, the diagnoses in a decreasing order of the number of TBI patients admitted were A-SDH (50.0%), others (18.3%), T-SAH (15.3%), C-ICH (9.6%), A-EDH (6.5%), and DAI (0.3%), and this result was slightly different from that reported in previous study including 2617 patients of all age groups registered in the KNTDBS in 2016 [
14]. In a previous study, although the incidence of A-SDH was the highest, its proportion was only 37.5%. Whereas A-EDH had a high percentage of 15.1, the proportion of T-SAH (14.4%) and C-ICH (9.8%) was similar in this study (
Fig. 2). Epidural hematoma is typically observed in children and young adults, but is rare in elderly people, because of the firm adherence of the dura to the inner table of the skull.
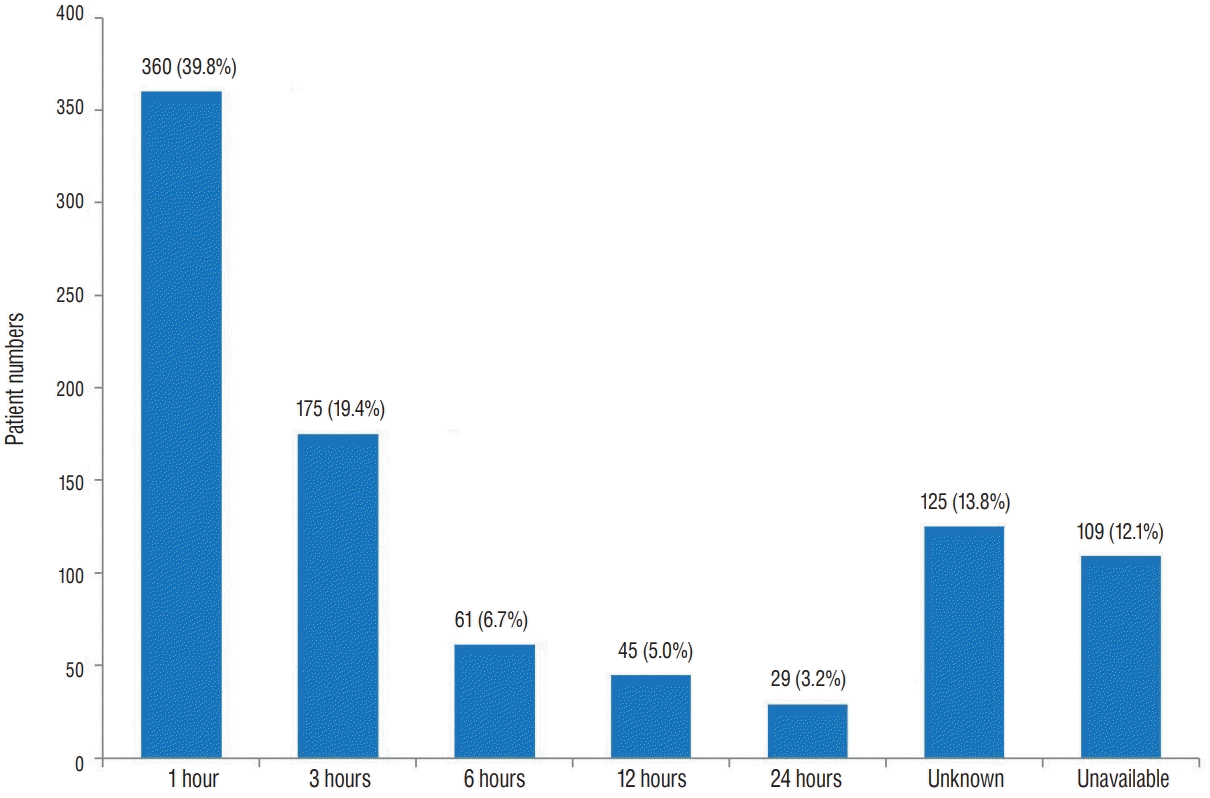
Fig. 3 shows that most patients arrived to the hospital within 1 hour of TBI, except those with unknown causes and unavailable data. These data suggest an excellent emergency patient transportation system and access to the hospitals of Korea.
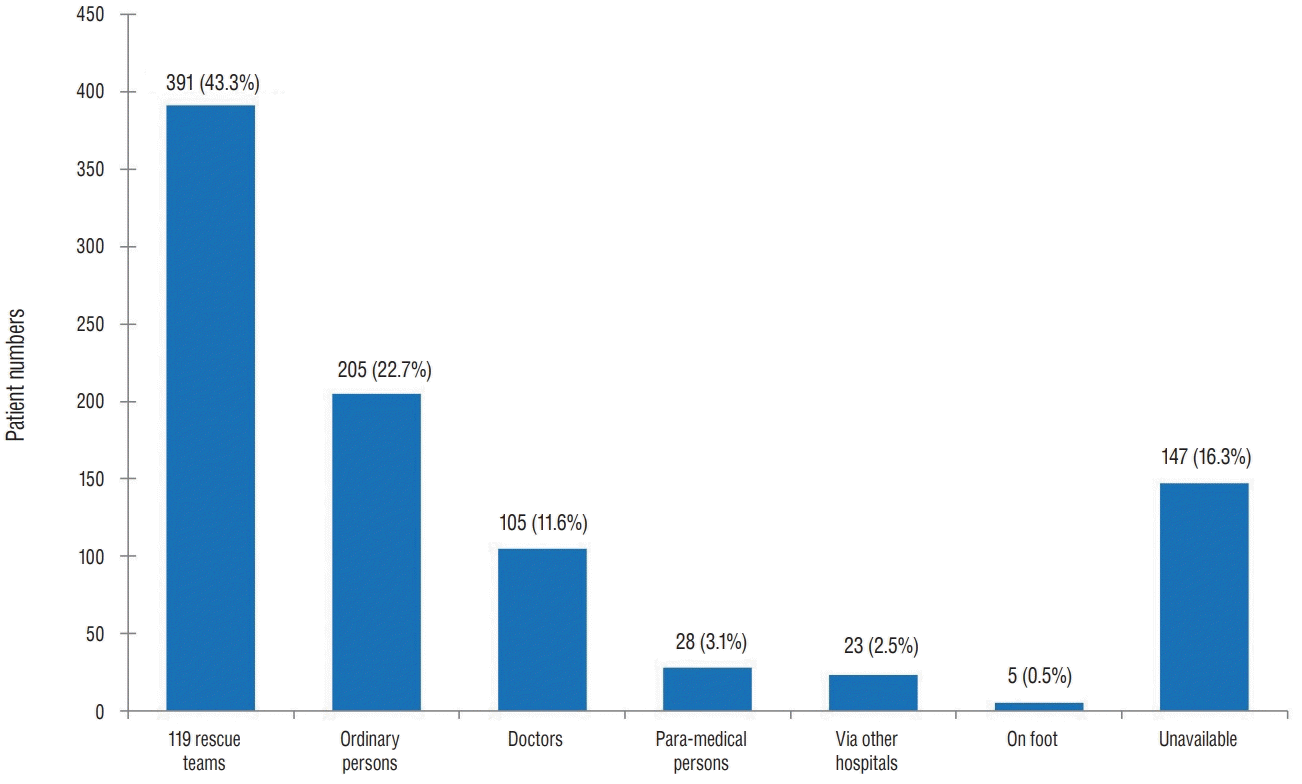
Fig. 4 shows that people who provided first aid earliest were 119 rescue team members, followed by ordinary people. Notably, ordinary people were at the second rank in providing emergency treatment, indicating that these people possess the knowledge of first aid to some extent and actively participated in patient treatment.
The proportion of surgical treatment was similar in both men (37.8%) and women (35.8%).
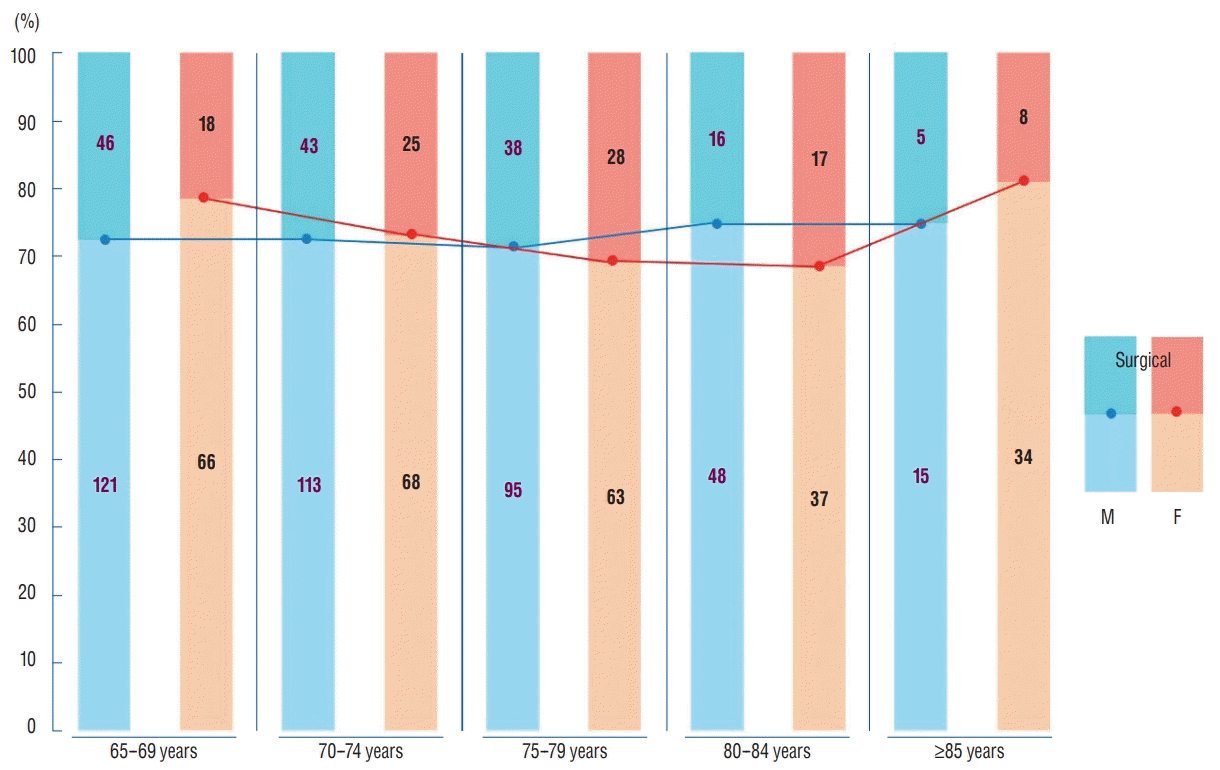
Fig. 5 shows that the similar proportions of surgical and non-surgical treatment of elderly patients with TBI for all age groups of both men and women. This suggests that recently, many surgeons are willing to perform surgery for elderly patients because of the advancements in modern medical science and prolonged average lifespans.
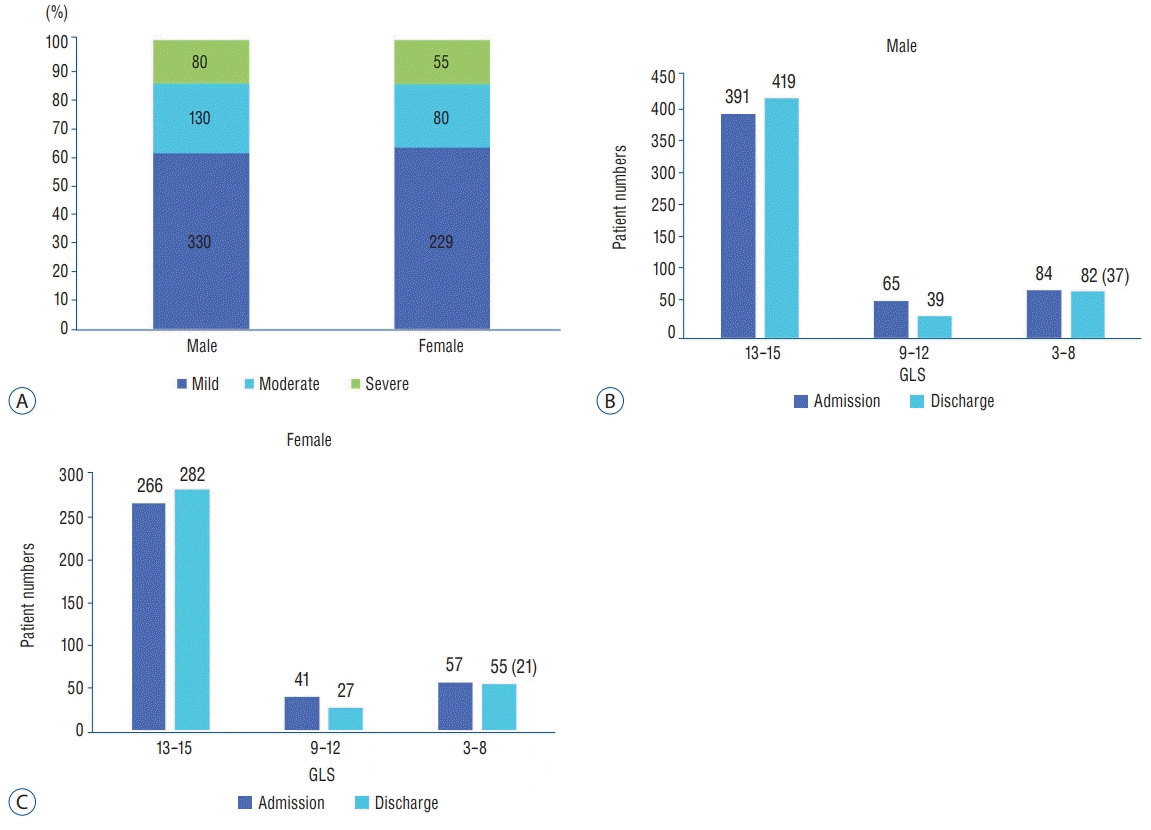
Fig. 6 shows that the severity of brain injury, GCS score at admission, and discharge rate showed similar incidence and proportions in both men and women. Most registered patients had mild brain injury (61.8%) and GCS 13–15 score at admission (72.7%). These proportions explain much lower mortality (6.42%) in this study compared with that in several studies in other countries with a high proportion of severe TBI on registered elderly patients [
8,
12,
13,
15].
Table 3 shows that the clinical characteristics of non-survivors and survivors and mortality. Older adults have the highest rates of hospitalization and mortality following TBI and experience poorer outcomes than do younger adults with the same injury severity [
16]. Age has been reported to be the single most predictive factor for poor prognosis, and geriatric TBI patients have been frequently shown to have greater mortality and worse outcomes than their younger counterparts [
7,
12,
15,
17]. Previous study of the KNTDBS confirmed these previously reported results of significantly higher mortality in patients older than 61 years [
14]. However, the mortality rate was not significantly different with respect to the age distribution of patients older than 65 years, although the mortality rate has rapidly increased since the age of 85 years. The mortality rate of male and female patients was not significantly different in this study, although risk factors for TBI among older adults may vary by sex. Albrecht et al. [
1] found no sex differences in mortality following isolated TBI among older adults, in contrast to other studies and their own analyses using all TBI cases. Leitgeb et al. [
10] also did not observe a significant effect of sex on mortality after adjustment for injury severity and mechanisms. In contrast, Davis et al. [
4] reported that post-menopausal women (age ≥50 years) were at a decreased risk of mortality at discharge compared with age-matched men. Berry et al. [
2] reported that post-menopausal women (age ≥55 years) were at a decreased risk of mortality compared with age-matched men. Among elderly adults, falls are the most common cause of TBI, representing 50–80% of injuries in this population [
3,
5,
6]. In this study, the mortality rate in the TA group (11.65%) was higher than that in the fall group (3.77%). The severity of brain injury (mild, moderate, and severe) showed a clear difference between fall (67.8%, 24.1%, and 8.0%, respectively) and TA (54.6%, 23.7%, and 21.7%, respectively) group suggesting that mortality was significantly higher in the TA group due to more grave conditions of patients. The author assume that the reasons for the difference in the mortality rate were comorbidities and injury severity according to the mechanisms of injury between falls and TA. Notably, injury severity is a significant predictor of mortality in elderly patients with TBI. Fu et al. [
6] showed that increased age, comorbidities, and injury severity were independent predictors of mortality; these findings are consistent with those previously reported. Utomo et al. [
18] found that severe TBI (GCS score, 3–8) was associated with a 24-fold increased risk of death compared with mild TBI (GCS score, 13–15). Similarly, McIntyre et al. [
11] analyzed 13 studies involving 35157 patients and reported a 12.7 times increased risk of death for severe TBI versus mild TBI.
Shimoda et al. [
13] reported that surgical management was associated with improved outcomes and mortality rate of elderly patients with TBI. This was particularly true among those who experienced acute subdural hematomas and those with GCS scores of 6–15, provided with intensive neurocritical care management although surgical management was not reported to be an effective treatment in elderly patients with GCS scores of 3–5. However, mortality in the non-surgery group was significantly lower than that in the surgery group. The reasons for this much differences with study of Shimoda et al. [
13], the proportion of severe (GCS, 3–8) was 15.6% and surgery group was 27.0% patient in our study, while the proportion of severe (GCS, 3–8) was 76.4% and surgery group was 44.1% patient in Shimoda et al.’s [
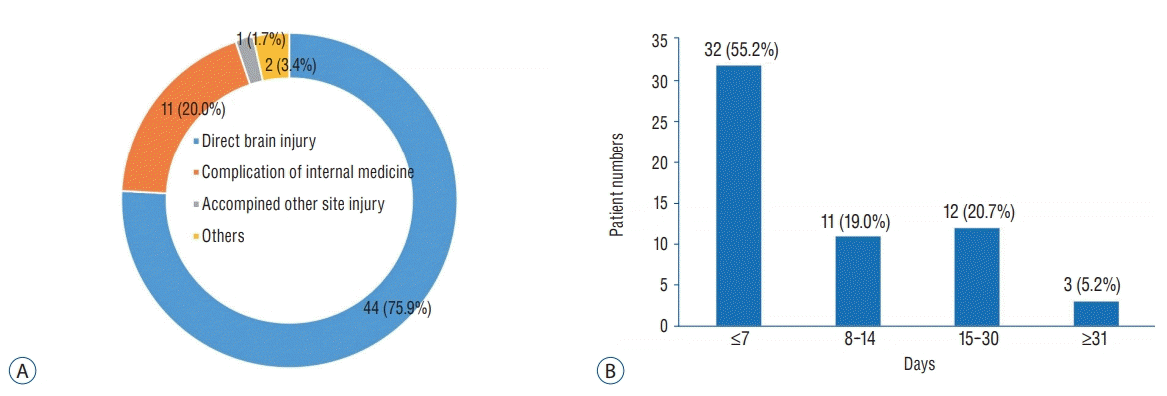
13] study. Among 125 hospitalized non-survivors, the proportions of the cause of death and the time interval from TBI onset to death was not much different with those reported in a previous study including all age groups [
14]. Most patients died of direct brain damage within 7 days (
Fig. 7).
The registered data provides a useful tool for quality control of the appropriate treatment plan for trauma because it can compare the medical practices of participating hospitals. In addition, trauma registration data is required to register the trauma mechanism and the environment at the time of injury, so that the data can be used well to lower the risk of similar type of trauma. However, different systems in each institute make the registered data inconsistent and unreliable. In addition, registering a trauma patient is costly and time-consuming, and requires a consistent registrant.
The study has some limitations as we noted in our previous because we used the same data bank system with the KNTDBS [
14]. In summary, 1) we could not identify the number of TBI patients who were not registered in the KNTDBS. 2) There is a lack of precise criteria and definitions of multiple or combined injuries in the diagnosis categories as well as in the diagnoses classified as others. 3) There are no precise criteria for the separate category of brain injury severity. 4) Because there is no precise unified surgical indication for TBI, each of the 20 institution is likely to have different surgical indication. And 5) there is a lack of various factors that affect outcomes and mortality rate. Therefore, we need more precise and detailed classification systems with respect to the causes of TBI in nonsurvivors to investigate the cause of death.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download