1. Bang WS, Kim KT, Cho DC, Kim HJ, Sung JK. Valproic Acid increases expression of neuronal stem/progenitor cell in spinal cord injury. J Korean Neurosurg Soc. 54:8–13. 2013.

2. Browne KD, Chen XH, Meaney DF, Smith DH. Mild traumatic brain injury and diffuse axonal injury in swine. J Neurotrauma. 28:1747–1755. 2011.

3. Donati G, Kapetanios A, Dubois-Dauphin M, Pournaras CJ. Caspaserelated apoptosis in chronic ischaemic microangiopathy following experimental vein occlusion in mini-pigs. Acta Ophthalmol. 86:302–306. 2008.

4. Jones CF, Lee JH, Burstyn U, Okon EB, Kwon BK, Cripton PA. Cerebrospinal fluid pressures resulting from experimental traumatic spinal cord injuries in a pig model. J Biomech Eng. 135:101005. 2013.

5. Jones CF, Lee JH, Kwon BK, Cripton PA. Development of a large-animal model to measure dynamic cerebrospinal fluid pressure during spinal cord injury: laboratory investigation. J Neurosurg Spine. 16:624–635. 2012.

6. Kim KT, Kim HJ, Cho DC, Bae JS, Park SW. Substance P stimulates proliferation of spinal neural stem cells in spinal cord injury via the mitogenactivated protein kinase signaling pathway. Spine J. 15:2055–2065. 2015.

7. Kim KT, Kim MJ, Cho DC, Park SH, Hwang JH, Sung JK, et al. The neuroprotective effect of treatment with curcumin in acute spinal cord injury: laboratory investigation. Neurol Med Chir (Tokyo). 54:387–394. 2014.

8. Kim KT, Nam TK, Park YS, Kim YB, Park SW. Neuroprotective effect of anthocyanin on experimental traumatic spinal cord injury. J Korean Neurosurg Soc. 49:205–211. 2011.

9. Kuluz JW, Prado R, He D, Zhao W, Dietrich WD, Watson B. New pediatric model of ischemic stroke in infant piglets by photothrombosis: acute changes in cerebral blood flow, microvasculature, and early histopathology. Stroke. 38:1932–1937. 2007.

10. Kwon BK, Borisoff JF, Tetzlaff W. Molecular targets for therapeutic intervention after spinal cord injury. Mol Interv. 2:244–258. 2002.

11. Kwon BK, Hillyer J, Tetzlaff W. Translational research in spinal cord injury: a survey of opinion from the SCI community. J Neurotrauma. 27:21–33. 2010.

12. Kwon BK, Roy J, Lee JH, Okon E, Zhang H, Marx JC, et al. Magnesium chloride in a polyethylene glycol formulation as a neuroprotective therapy for acute spinal cord injury: preclinical refinement and optimization. J Neurotrauma. 26:1379–1393. 2009.

13. Kwon BK, Streijger F, Fallah N, Noonan VK, Bélanger LM, Ritchie L, et al. Cerebrospinal fluid biomarkers to stratify injury severity and predict outcome in human traumatic spinal cord injury. J Neurotrauma. 34:567–580. 2017.

14. Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 4:451–464. 2004.

15. Lee JH, Jones CF, Okon EB, Anderson L, Tigchelaar S, Kooner P, et al. A novel porcine model of traumatic thoracic spinal cord injury. J Neurotrauma. 30:142–159. 2013.

16. Lee JH, Roy J, Sohn HM, Cheong M, Liu J, Stammers AT, et al. Magnesium in a polyethylene glycol formulation provides neuroprotection after unilateral cervical spinal cord injury. Spine (Phila Pa 1976). 35:2041–2048. 2010.

17. Mordasini P, Frabetti N, Gralla J, Schroth G, Fischer U, Arnold M, et al. In vivo evaluation of the first dedicated combined flow-restoration and mechanical thrombectomy device in a swine model of acute vessel occlusion. AJNR Am J Neuroradiol. 32:294–300. 2011.

18. Okon EB, Streijger F, Lee JH, Anderson LM, Russell AK, Kwon BK. Intraparenchymal microdialysis after acute spinal cord injury reveals differential metabolic responses to contusive versus compressive mechanisms of injury. J Neurotrauma. 30:1564–1576. 2013.

19. Rabchevsky AG, Fugaccia I, Sullivan PG, Scheff SW. Cyclosporin A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J Neurotrauma. 18:513–522. 2001.

20. Streijger F, Lee JH, Chak J, Dressler D, Manouchehri N, Okon EB, et al. The effect of whole-body resonance vibration in a porcine model of spinal cord injury. J Neurotrauma. 32:908–921. 2015.

21. Streijger F, Lee JH, Manouchehri N, Melnyk AD, Chak J, Tigchelaar S, et al. Responses of the acutely injured spinal cord to vibration that simulates transport in helicopters or mine-resistant ambush-protected vehicles. J Neurotrauma. 33:2217–2226. 2016.

22. Streijger F, Lee JH, Manouchehri N, Okon EB, Tigchelaar S, Anderson LM, et al. The evaluation of magnesium chloride within a polyethylene glycol formulation in a porcine model of acute spinal cord injury. J Neurotrauma. 33:2202–2216. 2016.

23. Streijger F, So K, Manouchehri N, Lee JHT, Okon EB, Shortt K, et al. Changes in pressure, hemodynamics and metabolism within the spinal cord during the first 7-days after injury using a porcine model. J Neurotrauma. 34:3336–3350. 2017.

24. Tator CH. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 59:957–982. discussion 982-987. 2006.
25. Teranishi K, Scultetus A, Haque A, Stern S, Philbin N, Rice J, et al. Traumatic brain injury and severe uncontrolled haemorrhage with short delay pre-hospital resuscitation in a swine model. Injury. 43:585–593. 2012.

26. Tigchelaar S, Streijger F, Sinha S, Flibotte S, Manouchehri N, So K, et al. Serum MicroRNAs reflect injury severity in a large animal model of thoracic spinal cord injury. Sci Rep. 7:1376. 2017.

27. Wernersson R, Schierup MH, Jørgensen FG, Gorodkin J, Panitz F, Staerfeldt HH, et al. Pigs in sequence space: a 0.66X coverage pig genome survey based on shotgun sequencing. BMC Genomics. 6:70. 2005.

28. Wofford KL, Harris JP, Browne KD, Brown DP, Grovola MR, Mietus CJ, et al. Rapid neuroinflammatory response localized to injured neurons after diffuse traumatic brain injury in swine. Exp Neurol. 290:85–94. 2017.

29. Zurita M, Aguayo C, Bonilla C, Otero L, Rico M, Rodríguez A, et al. The pig model of chronic paraplegia: a challenge for experimental studies in spinal cord injury. Prog Neurobiol. 97:288–303. 2012.

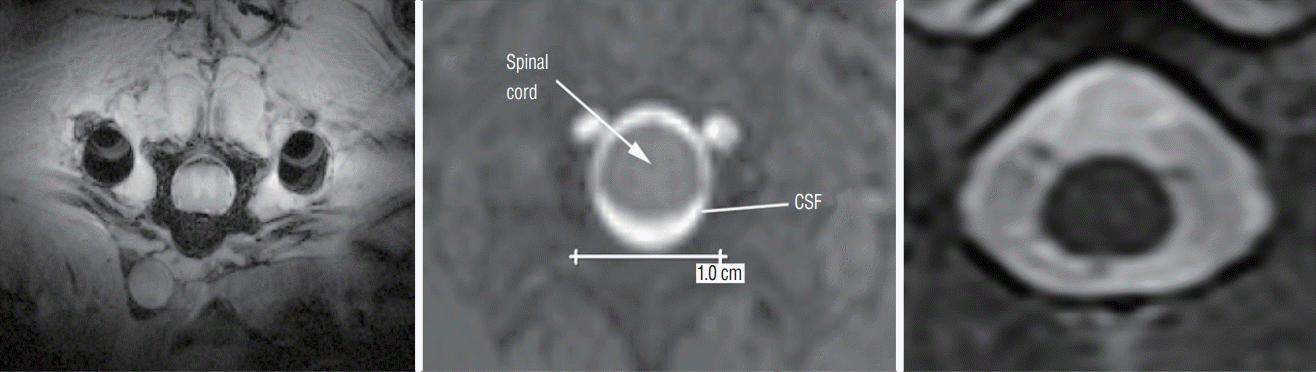
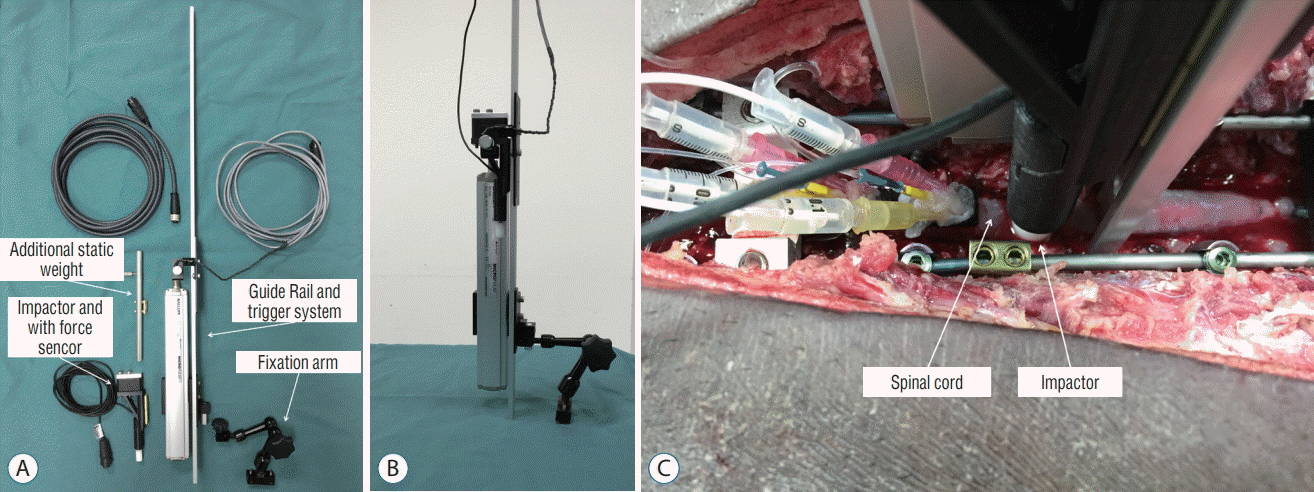
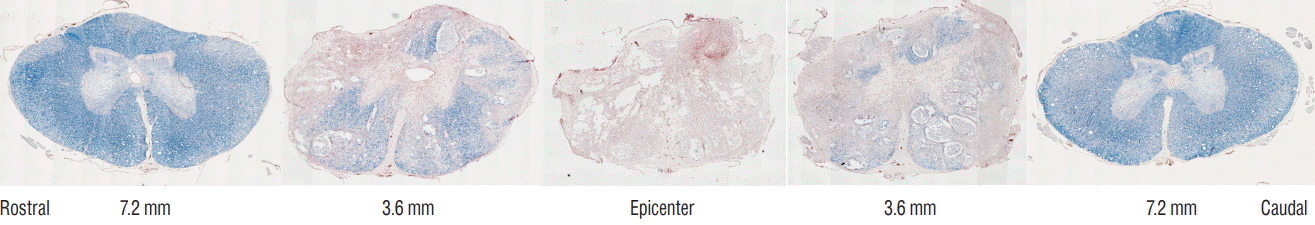
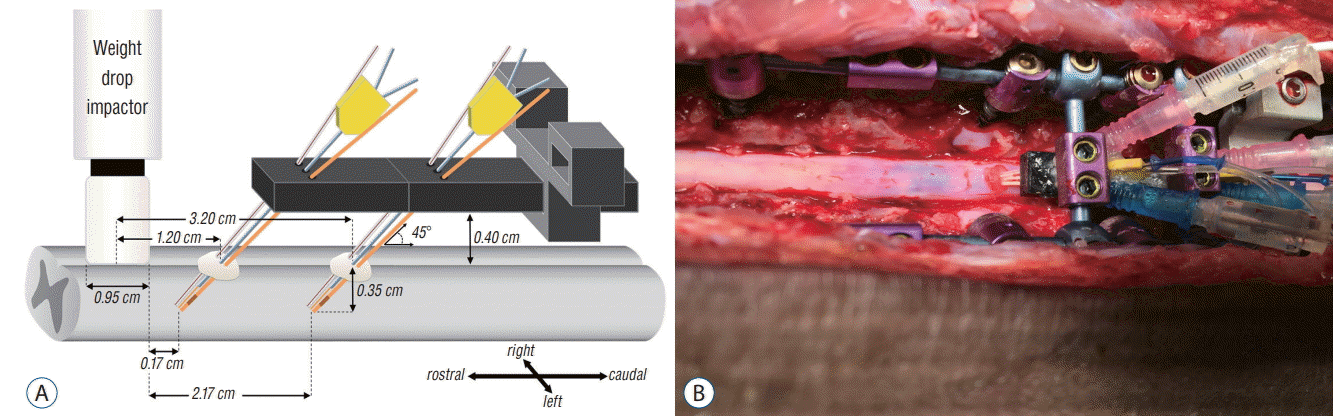




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download