Abstract
Objective
The aims in the management of thoracolumbar spinal fractures are not only to restore vertebral column stability, but also to obtain acceptable alignment of the thoracolumbar junction (T-L junction) to prevent complications. However, insufficient surgical correction of the thoracolumbar spine would be likely to cause late progression of abnormal kyphosis. Therefore, we identified the surgical factors that affected unfavorable radiologic outcomes of the thoracolumbar spine after surgery.
Methods
This study was conducted in a single institution from January 2007 to December 2013. A total of 98 patients with unstable thoracolumbar spine fracture were included. In these patients, fixation was done through transpedicular screws with rods by three surgical patterns. We reviewed digital radiographs and analyzed the images preoperatively and postoperatively during follow-up visits to compare the change of the thoracolumbar Cobb angle with radiologic parameters and clinical outcomes. The unfavorable radiologic group was defined as the patients who were measured as having greater than 20 degrees of thoracolumbar Cobb angle on the last follow-up, or who underwent kyphotic progression of thoracolumbar Cobb angle greater than 10 degrees from the immediate postoperative state to final follow-up, or who had overt instrument failure with/without additional surgery. We assessed the risk factors that affected the unfavorable radiologic outcomes.
Results
We had 43 patients with unfavorable radiologic outcomes, including 35 abnormal thoracolumbar alignments and 14 instrumental failures with/without additional surgery. The multivariate logistic regression test showed that immediate postoperative T-L junction Cobb angle less than 10.5 degrees was a statistically significant risk factor, as well as the presence of osteoporosis (p=0.017 and 0.049, respectively).
Conclusion
Insufficient correction of thoracolumbar kyphosis was considered to be a major factor of an unfavorable radiological outcome. The spinal surgeon should consider that having a T-L junction Cobb angle larger than 10.5 degrees immediately after surgery could result in an unfavorable radiological outcome, which is related to a poor clinical outcome.
Nearly 90% of all spinal fractures occur in the thoracolumbar region (T-L junction), and burst fractures comprise approximately 10% to 20% of such injuries, because of their biomechanical characteristics such as a transition between a rigid, kyphotic thoracic spine and a mobile, lordotic lumbar spine [10,12,15,21,27,31,40].
Although non-operative treatment for the relatively stable burst fracture in this region can be indicated, surgery is advocated for the unstable burst fracture. The advantages of surgery include better correction of kyphotic deformity, greater initial stability, an opportunity to perform direct or indirect decompression of neural elements, decreased requirements for an external immobilization, and an early return to work [1,10].
Although the ideal choice among three surgical approaches (i.e., anterior, posterior, or a combined approach) remains controversial, a high incidence of implant failure and correction loss even after stabilization surgery has been a major drawback in the posterior approach, despite its popularity and convenience to the spinal surgeon [9,11,14,16,17,20,26,28,29]. With a nearly 50% incidence of implant failure and 10 degrees of correction loss even after surgical reduction that is reported in the literature concerning the posterior approach, this approach also has an unfavorable radiological outcome of T-L junction burst fractures. In addition, the strong correlation between T-L junction Cobb angle greater than 20° through the correction loss and severe back pain have been shown in previous studies [2,9,14,18,29,32,37,38,43].
Thus, the authors reviewed T-L junction burst fracture patient data in a single center retrospectively to find risk factors and the cutoff values for the unfavorable radiological outcomes, such as the correction loss greater than 10 degrees, the development of postoperative final Cobb angle greater than 20 degrees, or early mechanical failure. In addition, we also analyzed the relation between clinical outcomes and the aforementioned unfavorable radiological outcomes.
Ninety-eight patients who had posterior fusion surgery for a single-level burst fracture at the T-L junction from T11 to L2 level between January 2001 and December 2012 at a single institution were included in the study. This study was reviewed and apporoved by the Institutional Review Board (GNAH IRB 201609009). The surgical indications for an acute, unstable burst fracture after high energy were as follows : 1) the presence of neurological deficit except an isolated partial nerve root problem, 2) a fracture with a Cobb angle of greater than 30 degrees, 3) a fracture with a compression rate of the anterior column greater than 40%, 4) a fracture with canal compromise greater than 50%, or 5) the presence of posterior ligamentous complex injury on magnetic resonance imaging [10].
Our inclusion criteria for this analysis were as follows : 1) a single-level burst fracture, 2) a posterior fusion surgery with only a pedicle screw, 3) no additional anterior surgery to support the anterior column, 4) no uncontrolled medical comorbidity, 5) a minimum follow-up time of more than 1 year after surgery, 6) no other serious combined injury, and 7) more than four points on the Thoracolumbar Injury Classification and Severity (TLICS) scale.
Surgical stabilization was done to correct trauma-induced kyphosis only through positional reduction and compression using a screw and rod system after general anesthesia. Because we included a posterior fusion surgery with only a pedicle screw for this analysis, there was no further procedure (e.g., an osteotomy or anterior graft support) for more correction of kyphosis except positional reduction in our study population. After pedicle screw insertion, an indirect decompression and bilateral compression maneuver using a screw and rod system was performed.
Three types of posterior fusion surgeries were performed randomly (Fig. 1). All the patients were advised to wear a thoracolumbosacral orthosis for 8 weeks postoperatively. An early rehabilitation and ambulation was recommended in all patients. Although bone mineral density (BMD) was not checked routinely, 20 patients older than 60 years of age or suspected to have poor bone quality (e.g., heavy smokers or scant bone marrow seen on computed tomography scan) had a BMD test performed after surgery. We defined osteoporosis as a T-score on the BMD test of less than -2.5. The bone materials that were used for fusion were detailed as three patterns (i.e., local bone only, local bone with allograft, or autograft iliac bone). We classified all patients’ postoperative ambulation status into two types including a standing with or without assistance and a wheelchair ambulation. Injury severity was measured with TLICS, and patients were classified into three groups according to the TLICS scores of 4–5, 6–8, and 9–10.
We defined the group of unfavorable radiological outcomes as the patients who were measured with greater than 20 degrees of thoracolumbar Cobb angle at the last follow-up, or who had kyphotic progression of thoracolumbar Cobb angle greater than 10 degrees from the immediate postoperative state to the 1-year follow-up, or who had overt instrument failure such as fracture, screw pullout followed by nonunion, or instability with or without additional revision surgery according to the previous guidelines [2,9,14,18,29,32,37,43]. The basic characteristics of all patients were compared between the two groups (i.e., favorable and unfavorable radiological outcomes) according to the age, gender, injury level, the presence of osteoporosis, operation type, bone fusion material, postoperative ambulation status, and preoperative TLICS score (Table 1).
Clinical outcomes including the low back outcome score (LBOS) and a change in the America Spinal Injury Association Impairment Scale (AIS) over 1 year were collected from medical records or follow-up visits and compared between groups. The grading system of LBOS was categorized through four levels : excellent (65–75), good (50–64), fair (30–49), and poor (0–30) [19]. We also analyzed whether or not a better (i.e., excellent or good) or worse (i.e., fair or poor) LBOS at 1 year after surgery was affected by radiological outcomes (i.e., favorable or unfavorable radiological outcome) [2,9,14,18,29,32,37,43]. AIS ranged from A (i.e., complete spinal cord injury) to E (i.e., neurologically intact status), and its change during 1 year was also compared between the favorable and unfavorable radiological outcome groups [25].
The Cobb angle at the T-L junction was defined as the angle between the superior endplate of T10 and the inferior endplate of L2. The Cobb angle before surgery, in the immediate postoperative period, and at 1 year postoperatively was measured (Fig. 2). The change of Cobb angle between preoperation and the immediate postoperative period and between the immediate postoperative period and 1 year postoperatively were also identified for statistical analysis.
The data are presented as means±standard deviations for continuous variables and as absolute or relative frequencies for categorical variables. Unpaired Student’s t tests or Mann-Whitney U tests were used to compare continuous variables, and the chi-square test was used for categorical variables. Multivariate logistic regression analyses using the aforementioned significant variables were used to identify independent predictors of favorable radiological outcomes. The results of a multivariate logistic regression were reported as odds ratios and 95% confidence intervals (CIs). Area under the receiver operating characteristics (ROC) curve was computed to determine an ideal cutoff value for preventing unfavorable radiological outcome using a significant variable in the multivariate regression test. The multivariate logistic regression analysis was done, again after changing the significant continuous variable of previous logistic regression to a categorical variable according to the above cutoff value. A p value ≤0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA).
There were 71 males and 27 females in our cohort, with a mean age of 48.0 years (range, 16–78 years). The mean followup duration was 23.6 months, with a range of 12 to 106 months. The 5, 19, 50, and 24 fractures were at T11, T12, L1, and L2 levels, respectively.
During the minimal 1-year follow-up, with a total of 98 patients, there were 35 patients (35.7%) with T-L junction Cobb angle greater than 20 degrees at the final follow-up visit, 22 patients (22.4%) with kyphotic progression of thoracolumbar cobb angle greater than 10 degrees, eight patients (8.1%) with instrument failure followed by revision surgery, and six patients (6.1%) with instrument failure without revision surgery. Because 28 patients were included in more than two of the previously mentioned categories, we classified 43 patients into the unfavorable radiological outcome group and the other 55 patients into the favorable radiological outcome group. There was a statistically significant difference among the basic characteristics between the two groups in age, sex, and osteoporosis (Table 1).
Among the 98 patients, the LBOS of 59 patients was fair or poor at the 1-year postoperative follow-up visit. There were 20 and 39 patients in the favorable and unfavorable radiological outcome groups, respectively. There was a statistically significant difference between the two groups (p=0.001). Moreover, the changed grade from preoperative AIS to that of 1-year postoperative follow-up was compared between groups. Also, 0, 1, 2, and 3 grading changes were observed in 19, 21, 14, and one patient in the favorable radiological outcome group, respectively, and 23, 17, three, and zero patients in the unfavorable radiological outcome group, respectively. There was no statistically significant difference between the two groups (Table 2).
The mean preoperative, immediate postoperative, and 1-year postoperative T-L junction Cobb angle were 15.32±6.90, 5.78±4.98, and 11.41±5.34 degrees, respectively, in the favorable radiological group. Those in the unfavorable radiological group were 19.09±7.96, 13.19±5.60, and 23.95±6.09 degrees. The changes of Cobb angle between preoperation and the immediate postoperative period (i.e., surgical correction) were 9.45±7.12 degrees in the favorable radiological outcome group and 6.05±6.00 degrees in the unfavorable radiological outcome group. The changes of Cobb angle between the immediate postoperative period and 1 year postoperatively (i.e., loss of correction) was 4.56±3.27 degrees in the favorable radiological outcome group and 10.91±5.61 degrees in the unfavorable radiological outcome group. There was a statistically significant difference in the immediate postoperative Cobb angle between the favorable and unfavorable radiological outcome groups (p=0.000, Table 3). Multivariate logistic regression test using statistically significant independent variables showed that an immediate postoperative T-L junction Cobb angle as a continuous variable was only a significant risk factor for an unfavorable radiological outcome (p<0.0001, Table 4). In ROC curve analysis for favorable radiological outcomes, the area under the ROC curves was 0.836 (95% CI 0.75–0.92), and compared to the other variables, immediate postoperative T-L junction Cobb angle (10.5 degrees) had the most reliable diagnostic power for predicting favorable radiological outcomes. The sensitivity and specificity were 82% and 72%, respectively (Fig. 3). After changing a continuous variable of immediate postoperative T-L junction Cobb angle into a categorical variable according to 10.5 degrees, a multivariate logistic regression test also showed immediate postoperative T-L junction Cobb angle less than 10.5 degrees was a statistically significant risk factor as well as the presence of osteoporosis (p=0.017 and 0.049, respectively; Table 5).
Although surgical treatment for T-L junction burst fractures is popularly performed, there has been no standard guideline regarding the surgical approach, fusion level, or the amount of kyphosis correction [8]. Which surgical approach (i.e., anteriorly, posteriorly, or combined anterior and posteriorly) is best has long been a matter of debate, because each of the three approaches has its own advantages and disadvantages. The posterior approach of the T-L junction is well established, with advantages such as more safety in exploring the surgical site without violating the pulmonary, visceral, and vascular structures and being less technically demanding [30]. However, an instrument failure and recurrence of kyphosis have been well reported when the posterior alone approach is made without vertebral body reconstruction, as in our procedure [9,14,29]. A suggested modality to solve these problems is long segment pedicle screw fixation (two above and two below); however, this procedure comes with less preservation of spinal motion and has been proven to be not significantly effective for preventing postoperative correction loss like our data [13].
One recent study reported that 12-degree T-L junction Cobb angle could be related to poor functional outcome. Because nearly 5 degrees of correction loss developed even after anterior-posterior surgery in this study, it could be suggested that the immediate postoperative Cobb angle should be less than 7 degrees for the reasonable functional outcome [35]. This value is a more strict cutoff value compared with our data, because this analysis is based on the functional outcome; our analysis was based on the radiological outcome.
It has been well known that a correction loss of nearly 10 degrees develops even after surgery and, if the kyphotic correction loss finally reaches 20 degrees Cobb angle, this amount has been related to poor function outcome [2,9,14,18,29,32,35,37,38,43]. Based on those hypotheses, we take it for granted that the immediate postoperative T-L junction Cobb angle should be greater than 10.5 degrees, not to reach 20 degrees of Cobb angle in our result. Interestingly, correction loss in the favorable radiological outcome group was less than that for the unfavorable radiological group, as well as the amount of immediate postoperative kyphosis (Table 3). Considering this result (Table 3) and multivariate logistic regression analysis (Table 4) simultaneously, we believe that the ideal immediate postoperative T-L junction angle is no more than 10.5 degrees. This angle could give us less correction loss, and is similar to the amount of correction loss after surgery of a previous article [35].
In the current study, we found that most patients’ T-L junction kyphosis had been corrected immediately postoperatively, but a considerable number of patients had progressive worsening of their corrected thoracolumbar alignment during a period of less than 1 year after fusion surgery. Interestingly, this tendency was significantly observed in patients with severe preoperative T-L junction kyphosis, and they also underwent a relatively small amount of surgical correction in their T-L junction kyphosis. The biomechanical characteristics of the thoracolumbar spine could be one of the probable explanations of this tendency. When an increased load is given to the thoracic curvature, the lower sections of the spine present a proportionally greater amount of body mass shifting, anteriorly without the load sharing by the sternum or ribs [5,6]. Likewise, a return to the normal range of thoracolumbar curvature after unstable fracture is also important to avoid additional excessive loading to the spine, which might be another secondary complication [23].
The clinical outcomes were evaluated by LBOS and AIS in this study, and we experienced that the poor outcome of the unfavorable radiological outcome group was related to a poor clinical outcome, such as in the LBOS; conversely, AIS was not significantly correlated to the radiological outcome. This discrepancy was inferred from chronic sustained back pain, caused by malalignment of the thoracolumbar spine (i.e., kyphosis) even with neurological improvement. Although the discrepancy of radiological outcomes and clinical outcomes are often reported by some articles [3,34,41-43], some other evidence also reported that T-L junction kyphosis led to chronic back pain and that its correction resulted in the improvement of clinical symptoms in the posttraumatic kyphosis [7,39,44].
We suggest the value of T-L junction Cobb angle less than 10.5 degrees for the reasonable extent of surgical correction in thoracolumbar burst fracture through this study. However, we also believe that it is difficult to make an exact T-L junction angle with such a narrow guideline during surgery. Thus, our recommendation is that a surgeon should keep in mind that the larger the immediate postoperative T-L junction kyphosis, the larger the possible correction loss, resulting in poor clinical outcome. With this guideline in mind, when we found an abnormally uncorrected kyphosis, especially one greater than 10.5 degrees even after a positional reduction, more aggressive osteotomy or anterior graft support with a partial corpectomy could be recommended. A combined anterior and posterior procedure could also be an option for the surgical treatment of severe kyphotic deformities [4,36].
For a patient with a presence of osteoporosis is another significant risk factor (Table 5). The poor bone quality in these patients is another probable cause of the progression of thoracolumbar kyphosis and instrument failure during followup [22,24,33]. Thus, we also recommend more careful concern be given within our cutoff value for patients with osteoporosis.
Among the limitations of our study, we had performed the stabilization through the posterior approach with only pedicle screws. Therefore, the previously mentioned extent of tolerable surgical thoracolumbar kyphosis was not the absolute value, but it would be a helpful reference. In addition, our patients did not undergo whole spinal radiographic examination; thus, there is another limitation for evaluating the relation between the T-L junction kyphosis and global sagittal parameters. Further studies without the confounding factors and a larger cohort are warranted for more accurate results.
Insufficient correction of thoracolumbar kyphosis was considered to be a major factor of an unfavorable radiological outcome. Although unfavorable radiological outcomes did not affect more neurological improvement, they significantly affected LBOS. Thus, the spinal surgeon should consider that a remaining T-L junction kyphotic Cobb angle greater than 10.5 degrees immediately after surgery could result in an unfavorable radiological outcome, which is related to poor clinical outcome.
References
1. Akbarnia BA, Crandall DG, Burkus K, Matthews T. Use of long rods and a short arthrodesis for burst fractures of the thoracolumbar spine. a long-term follow-up study. J Bone Joint Surg Am. 76:1629–1635. 1994.

2. Alanay A, Acaroglu E, Yazici M, Oznur A, Surat A. Short-segment pedicle instrumentation of thoracolumbar burst fractures: does transpedicular intracorporeal grafting prevent early failure? Spine (Phila Pa 1976). 26:213–217. 2001.
3. Albayrak A, Balioglu MB, Misir A, Kargin D, Tacal MT, Atici Y, et al. Preoperative and postoperative photographs and surgical outcomes of patients with kyphosis. Spine (Phila Pa 1976). 41:E1185–E1190. 2016.

4. Been HD, Bouma GJ. Comparison of two types of surgery for thoracolumbar burst fractures: combined anterior and posterior stabilisation vs. posterior instrumentation only. Acta Neurochir (Wien). 141:349–357. 1999.

5. Briggs AM, van Dieën JH, Wrigley TV, Greig AM, Phillips B, Lo SK, et al. Thoracic kyphosis affects spinal loads and trunk muscle force. Phys Ther. 87:595–607. 2007.

6. Bruno AG, Anderson DE, D’Agostino J, Bouxsein ML. The effect of thoracic kyphosis and sagittal plane alignment on vertebral compressive loading. J Bone Miner Res. 27:2144–2151. 2012.

7. Buchowski JM, Kuhns CA, Bridwell KH, Lenke LG. Surgical management of posttraumatic thoracolumbar kyphosis. Spine J. 8:666–677. 2008.

8. Cahueque M, Cobar A, Zuñiga C, Caldera G. Management of burst fractures in the thoracolumbar spine. J Orthop. 13:278–281. 2016.

9. Carl AL, Tromanhauser SG, Roger DJ. Pedicle screw instrumentation for thoracolumbar burst fractures and fracture-dislocations. Spine (Phila Pa 1976). 17(8 Suppl):S317–S324. 1992.

10. Dai LY, Jiang SD, Wang XY, Jiang LS. A review of the management of thoracolumbar burst fractures. Surg Neurol. 67:221–231. discussion 231. 2007.

11. Daniaux H, Seykora P, Genelin A, Lang T, Kathrein A. Application of posterior plating and modifications in thoracolumbar spine injuries. Indication, techniques, and results. Spine (Phila Pa 1976). 16(3 Suppl):S125–S133. 1991.
12. Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 8:817–831. 1983.

13. Dobran M, Nasi D, Brunozzi D, di Somma L, Gladi M, Iacoangeli M, et al. Treatment of unstable thoracolumbar junction fractures: short-segment pedicle fixation with inclusion of the fracture level versus long-segment instrumentation. Acta Neurochir (Wien). 158:1883–1889. 2016.

14. Ebelke DK, Asher MA, Neff JR, Kraker DP. Survivorship analysis of VSP spine instrumentation in the treatment of thoracolumbar and lumbar burst fractures. Spine (Phila Pa 1976). 16(8 Suppl):S428–S432. 1991.

15. Esses SI, Botsford DJ, Kostuik JP. Evaluation of surgical treatment for burst fractures. Spine (Phila Pa 1976). 15:667–673. 1990.

16. Esses SI, Botsford DJ, Wright T, Bednar D, Bailey S. Operative treatment of spinal fractures with the AO internal fixator. Spine (Phila Pa 1976). 16(3 Suppl):S146–S150. 1991.

17. Gertzbein SD. Scoliosis research society. Multicenter spine fracture study. Spine (Phila Pa 1976). 17:528–540. 1992.
18. Gertzbein SD, Court-Brown CM, Marks P, Martin C, Fazl M, Schwartz M, et al. The neurological outcome following surgery for spinal fractures. Spine (Phila Pa 1976). 13:641–644. 1988.

19. Greenough CG, Fraser RD. Assessment of outcome in patients with low-back pain. Spine (Phila Pa 1976). 17:36–41. 1992.

20. Haas N, Blauth M, Tscherne H. Anterior plating in thoracolumbar spine injuries. indication, technique, and results. Spine (Phila Pa 1976). 16(3 Suppl):S100–S111. 1991.

21. Hu R, Mustard CA, Burns C. Epidemiology of incident spinal fracture in a complete population. Spine (Phila Pa 1976). 21:492–499. 1996.

22. Josten C, Schmidt C, Spiegl U. Osteoporotic vertebral body fractures of the thoracolumbar spine. Diagnostics and therapeutic strategies. Chirurg. 83:866–874. 2012.
23. Khoueir P, Oh BC, Wang MY. Delayed posttraumatic thoracolumbar spinal deformities: diagnosis and management. Neurosurgery. 63(3 Suppl):117–124. 2008.
24. Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J. 6:479–487. 2006.

25. Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 34:535–546. 2011.

26. Knop C, Fabian HF, Bastian L, Blauth M. Late results of thoracolumbar fractures after posterior instrumentation and transpedicular bone grafting. Spine (Phila Pa 1976). 26:88–99. 2001.

27. Kraemer WJ, Schemitsch EH, Lever J, McBroom RJ, McKee MD, Waddell JP. Functional outcome of thoracolumbar burst fractures without neurological deficit. J Orthop Trauma. 10:541–544. 1996.

28. Lindsey RW, Dick W. The fixateur interne in the reduction and stabilization of thoracolumbar spine fractures in patients with neurologic deficit. Spine (Phila Pa 1976). 16(3 Suppl):S140–S145. 1991.

29. McLain RF, Sparling E, Benson DR. Early failure of short-segment pedicle instrumentation for thoracolumbar fractures. A preliminary report. J Bone Joint Surg Am. 75:162–167. 1993.

30. Mermelstein LE, McLain RF, Yerby SA. Reinforcement of thoracolumbar burst fractures with calcium phosphate cement. A biomechanical study. Spine (Phila Pa 1976). 23:664–670. discussion 670-671. 1998.

31. Müller U, Berlemann U, Sledge J, Schwarzenbach O. Treatment of thoracolumbar burst fractures without neurologic deficit by indirect reduction and posterior instrumentation: bisegmental stabilization with monosegmental fusion. Eur Spine J. 8:284–289. 1999.

32. Parker JW, Lane JR, Karaikovic EE, Gaines RW. Successful shortsegment instrumentation and fusion for thoracolumbar spine fractures: a consecutive 41/2-year series. Spine (Phila Pa 1976). 25:1157–1170. 2000.

33. Paxinos O, Tsitsopoulos PP, Zindrick MR, Voronov LI, Lorenz MA, Havey RM, et al. Evaluation of pullout strength and failure mechanism of posterior instrumentation in normal and osteopenic thoracic vertebrae. J Neurosurg Spine. 13:469–476. 2010.

34. Rojas-Tomba F, Hernández-Ruiz Á, Menéndez-Quintanilla I, García de Quevedo-Puerta D, Moriel-Durán J, Villanueva-Pareja F. Radiologic and functional outcomes in unstable thoracolumbar fractures treated with short-segment pedicle instrumentation. Clin Spine Surg. 30:459–465. 2017.

35. Schulz R, Melcher RP, Garib MC, Schulz H, Weissman K, Harms J. Does kyphotic deformity correlate with functional outcomes in fractures at the thoracolumbar junction treated by 360° instrumented fusion? Eur J Orthop Surg Traumatol. 24 Suppl 1:s93–s101. 2014.

36. Song KS, Chang BS, Yeom JS, Lee JH, Park KW, Lee CK. Surgical treatment of severe angular kyphosis with myelopathy: anterior and posterior approach with pedicle screw instrumentation. Spine (Phila Pa 1976). 33:1229–1235. 2008.
37. Tezeren G, Kuru I. Posterior fixation of thoracolumbar burst fracture: short-segment pedicle fixation versus long-segment instrumentation. J Spinal Disord Tech. 18:485–488. 2005.
38. Tropiano P, Huang RC, Louis CA, Poitout DG, Louis RP. Functional and radiographic outcome of thoracolumbar and lumbar burst fractures managed by closed orthopaedic reduction and casting. Spine (Phila Pa 1976). 28:2459–2465. 2003.

39. Vaccaro AR, Silber JS. Post-traumatic spinal deformity. Spine (Phila Pa 1976). 26(24 Suppl):S111–S118. 2001.

40. Vaccaro AR, Kim DH, Brodke DS, Harris M, Chapman JR, Schildhauer T, et al. Diagnosis and management of thoracolumbar spine fractures. Instr Course Lect. 53:359–373. 2004.

41. Wang XB, Lü GH, Li J, Wang B, Lu C, Phan K. Posterior distraction and instrumentation cannot always reduce displaced and rotated posterosuperior fracture fragments in thoracolumbar burst fracture. Clin Spine Surg. 30:E317–E322. 2017.

42. Wood KB, Buttermann GR, Phukan R, Harrod CC, Mehbod A, Shannon B, et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit: a prospective randomized study with follow-up at sixteen to twenty-two years. J Bone Joint Surg Am. 97:3–9. 2015.

Fig. 1.
Lateral plain postoperative radiographs of three patients with L1 burst facture treated by three surgical techniques (A-C).
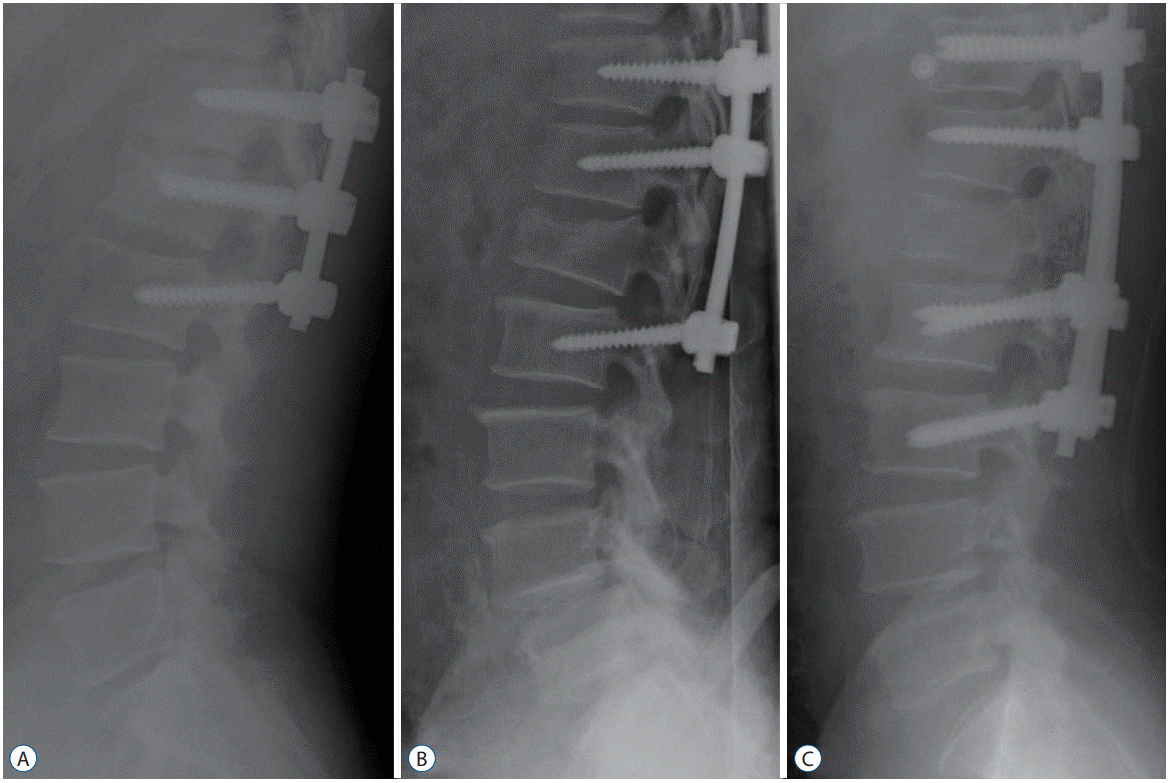
Fig. 2.
Postoperative radiographs showing the overall thoracolumbar Cobb angle for a case from each of the radiologic groups (left, unfavorable radiological outcome group; right, favorable radiological outcome group), which is a measured angle A between the superior endplate of T10 and the inferior endplate of L2.
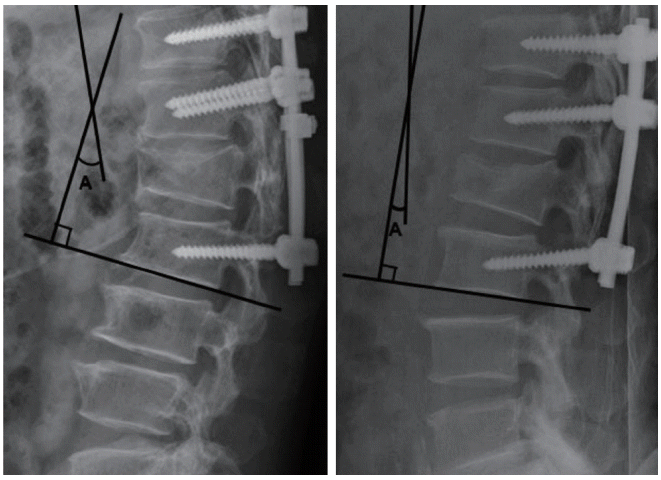
Fig. 3.
The receiver operating characteristics (ROC) curve analysis for favorable radiological outcomes, the area under the ROC curves was 0.836 (95% confidence interval 0.75–0.92). The Cutoff value of immediate postoperative T-L junction Cobb angle is 10.5 degrees (arrow). The sensitivity and specificity were 82% and 72%, respectively.
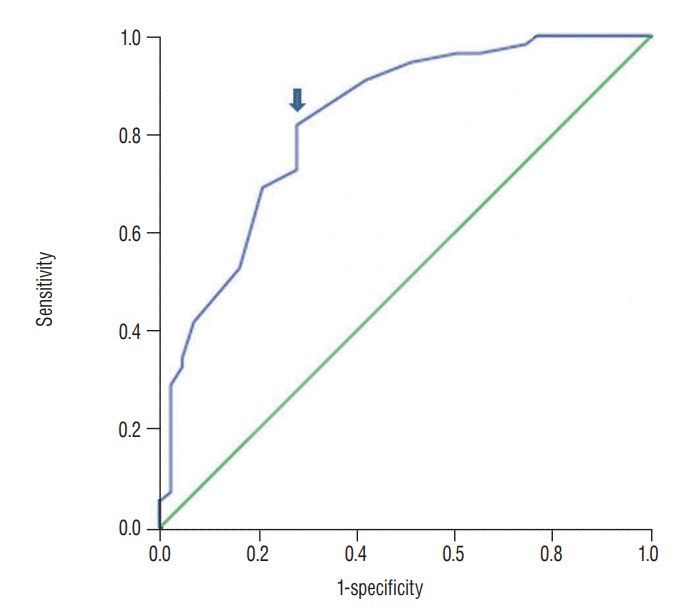
Table 1.
Comparison of baseline characteristics of the patients within favorable and unfavorable radiologic outcomes
| Favorable (n=55) | Unfavorable (n=43) | p-value | |
|---|---|---|---|
| Age (years) | 44.0±14.42 | 52.0±12.32 | 0.004* |
| Sex (M : F) | 34 : 21 | 37 : 6 | 0.008* |
| Injury level | 0.797 | ||
| T11 | 2 (4) | 3 (7) | |
| T12 | 10 (18) | 9 (21) | |
| L1 | 28 (51) | 22 (51) | |
| L2 | 15 (27) | 9 (21) | |
| Overt osteoporosis | 6 (11) | 14 (33) | 0.007* |
| Surgical method (fusion) | 0.246 | ||
| 2-level fusion | 35 (64) | 34 (79) | |
| 3-level fusion | 12 (22) | 5 (12) | |
| 4-level fusion | 8 (14) | 4 (9) | |
| Bone grafting | 0.791 | ||
| Local only | 8 (15) | 7 (16) | |
| Local+allograft | 41 (75) | 33 (77) | |
| Local+autograft (iliac) | 6 (10) | 3 (7) | |
| Postoperative ambulation | 0.453 | ||
| Standing independently | 42 (76) | 36 (84) | |
| Wheelchair assisted | 13 (24) | 7 (16) | |
| TLICS | 0.117 | ||
| 4–5 | 24 (44) | 26 (60) | |
| 6–8 | 14 (25) | 11 (26) | |
| 9–10 | 17 (31) | 6 (14) |
Table 2.
Comparison of clinical outcomes of the patients within favorable and unfavorable radiologic outcomes
| Clinical outcome | Favorable (n=55) | Unfavorable (n=43) | p-value |
|---|---|---|---|
| The low back outcome score | 0.001* | ||
| Poor or fair | 20 | 39 | |
| Excellent or good | 35 | 4 | |
| Initial AIS (A–E) | 0.569 | ||
| A | 6 | 2 | |
| B | 6 | 2 | |
| C | 11 | 10 | |
| D | 16 | 13 | |
| E | 16 | 16 | |
| The changed grading numbers from initial AIS of injured neurologic patients after surgery | 0.056 | ||
| 0 (no change) | 19 | 23 | |
| 1 (A→B; B→C; C→D; D→E) | 21 | 17 | |
| 2 (A→C; B→D) | 14 | 3 | |
| 3 (A→D) | 1 | 0 |
Table 3.
Comparison of radiologic parameters of the patients within favorable and unfavorable radiologic outcomes
Table 4.
Factors associated with favorable radiologic outcomes by multiple logistic regression analysis
Table 5.
Factors associated with favorable radiologic outcomes by multiple logistic regression analysis




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download